- LOGIN
- MemberShip
- 2025-12-26 17:26:02
- HER2 targeted therapy market exceeds KRW 200 billion
- by An, Kyung-Jin | translator Byun Kyung A | 2021-03-15 06:28:24
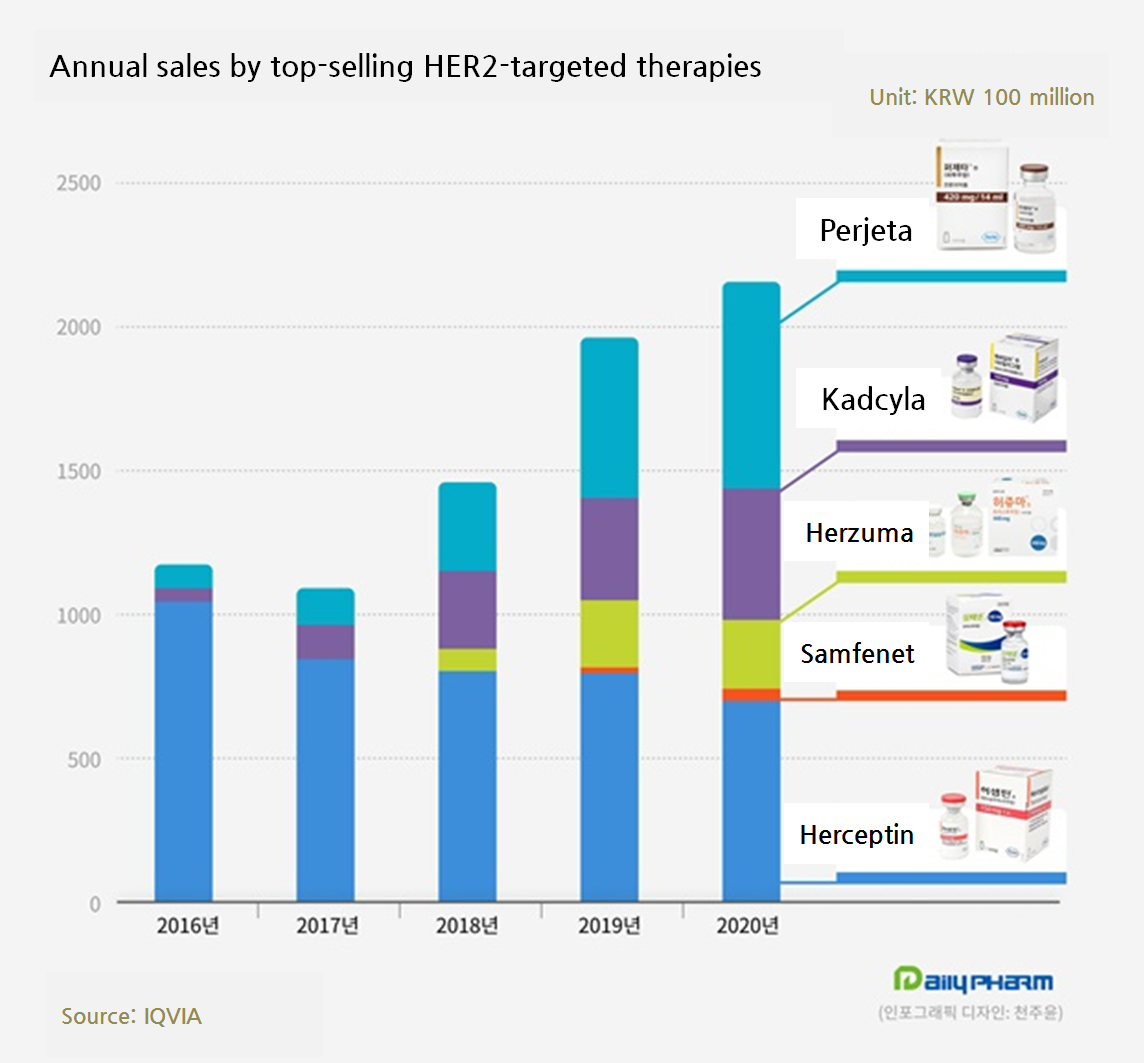
The South Korean market for targeted therapy prescribed to treat human epidermal growth factor receptor 2 (HER2)-positive breast cancer has exceeded 200 billion won in sales last year.
Two domestically developed biosimilars increased the market influence and made up for the sale revenue void in the original by the original maker’s follow-on drugs, such as Kadcyla and Perjeta, breaking the market sales record.
On Mar.
11, a pharmaceutical market research firm IQVIA reported the trastuzumab drug market size last year dropped to 99 billion won by year-on-year 4.8 percent.
The market size increased for two consecutive years since it plummeted from 103.4 billion won in 2016 to 83.6 billion won in 2017, and recovered up to annual sales of 100 billion in 2019.
But it took a slight fall after a year.
The drug is prescribed to HER2-positive patients with metastatic breast cancer and stomach cancer.
In South Korea, two biosimilars of the original Herceptin—Celltrion’s Herzuma and Samsung Bioepis’ Samfenet—were released to the market after the original’s patent expired.
The original generated sales of 69.9 billion won last year.
Compared against the year before, it was dropped by 11.2 percent.
And compared against 103.4 billion won raised in 2016, before the biosimilars were released, one-third of the sales were evaporated.
Herceptin’s pricing was brought down by 20 percent, from 517,628 won to 414,103 won per 150 mg injection, two months after Celltrion’s Herzuma was listed for National Health Insurance (NHI) reimbursement in April 2017.
And with another pricing drop, the original drug is currently maintaining the pricing at 362,340 won since April last year.
By principle of the South Korean drug pricing system, a biosimilar can be priced at 70 percent of the original’s pricing before the patent expiration.
And from October 2016, an item ‘developed by a company recognized as an innovative pharmaceutical company or a company with collaborative deal between South Korean and global companies, released first time internationally in South Korea, or manufactured in South Korea’ can be priced up to 80 percent of the original.
Meanwhile, an off-patent original’s pricing is reduced to 70 percent to 80 percent of the initial pricing when a biosimilar is launched.
The trastuzumab drug market shrunk down to 83.6 billion won due the pricing reduction in Herceptin in 2017.
But as biosimilars developed with South Korean technology pushed the sales, the market growth is catching up with the original’s gap.
Celltrion’s Herzuma sales last year grew by 10.4 percent from the year before and generated 25.4 billion won.
The Ministry of Food and Drug Safety (MFDS) authorized the sales of Herzuma in January 2014 and the drug started making sales revenue from the third quarter of 2017.
The 2018 sales was stale with 7.7 billion won, but the sales surged to 23 billion won in 2019 and it is keeping the growth ever since.
However, the domestic market share of Samsung Bioepis’ Samfenet is still insignificant.
Samfenet generated 3.6 billion won last year.
Although the sales grew by 65.8 percent compared to the year before, the gap with the competitor is rather substantial.
Currently, Daewoong Pharmaceutical is in charge of Samfenet sales.
Within three years of releasing the biosimilars, their market share in the trastuzumab drug market grew exponentially.
The two biosimilars took 29.3 percent of the last year’s trastuzumab drug market sales.
Herzuma’s share is at 25.7 percent, when Samfenet took up 3.6 percent.
Basically, Herzuma led the biosimilar market expansion.
And the targeted therapy market for the HER2-positive breast cancer patients expanded vastly as Herceptin developer Roche released follow-on drugs Kadcyla (trastuzumab emtansine) and Perjeta (pertuzumab).
Around 15 percent to 20 percent of the total breast cancer patients are confirmed positively with HER2, which has fast relapse and short survival period.
Roche’s Kadcyla generated 43.5 billion won last year, growing 22.7 percent from the year before.
Kadcyla is the first antibody drug conjugate (ADC) Roche first show cased for the breast cancer sector.
The drug can be prescribed to treat unresectable locally advanced or metastatic breast cancer in patients previously treated with trastuzumab and taxane.
During the same time, Roche’s Perjeta generated 74.1 billion won.
Growing 33.1 percent compared to the year before, the sales surpassed Herceptin for the first time.
The drug, in combination with docetaxel and trastuzamab, can be prescribed to patients with HER2-positive metastatic or locally recurrent unresectable breast cancer, who have not received previous HER2 targeted therapy or chemotherapy.
As it was listed for selective reimbursement in May 2019, the combination therapy with trastuzumab became the standard adjuvant treatment before a surgery.
Five drugs including the three trastuzumab drugs, Kadcyla and Perjeta, have raised 216.6 billion last year together and increased the market by 11.0 percent from the year before.
Compared to 116.1 billion won in 2016, the market sales have almost doubled in four years and broke the record.
The industry evaluates Roche has overcome off-patent Herceptin’s sales drop with the follow-on drugs.
Also the HER2-positive breast cancer patients would benefit from the expanded treatment options.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









