- LOGIN
- MemberShip
- 2025-12-26 12:17:58
- Sales of EGFR anticancer drug Tagrisso have been put on hold
- by An, Kyung-Jin | translator Choi HeeYoung | 2021-05-27 05:56:06
The market for EGFR lung cancer drugs has been put on hold.
Sales of Tagrisso, a third-generation drug, have declined since its benefit.
As new products emerge one after another, the market is expected to change within this year.
According to IQVIA, a pharmaceutical research institute, the size of Korea's market for EGFR tyrosine kinase inhibitors (TKI) in the first quarter of last year was ₩35.5 billion, down 2.9% from ₩36.5 billion a year earlier.
South Korea's EGFR-TKI market increased to ₩38.9 billion in sales in third quarter of last year as it continued to grow since first quarter of 2017, but fell for second consecutive quarter.

EGFR mutations are a very common mutant type observed in 30–40% of non-small cell lung cancers that account for 80–85% of lung cancer, occurring between exon 18 and exon 21.
It is known that Asian markets, including Korea, China, and Japan, are larger because they are more popular than Westerners.
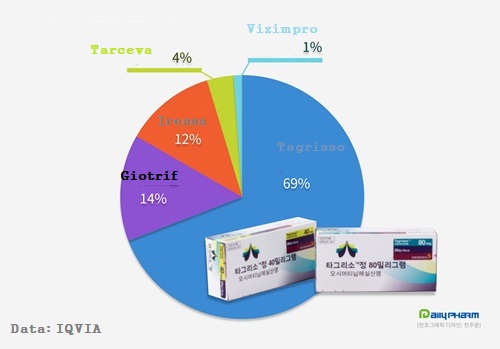
EGFR-TKI, which is currently on the market in South Korea, includes first-generation drugs Iressa (Gefitinib), Tarceva (Erlotinib), second-generation Giotrif (Afatinib), and third-generation Tagrisso (Osimertinib).
Among the five products, the total size of the market tends to depend on the sales of Tagrisso.
Tagrisso's first-quarter sales rose 3.1% year-on-year to ₩24.4 billion.
As of first quarter, Tagrisso accounts for 68.9% of all markets.
It is maintaining a four-fold gap with Giotrif, the second-largest item in sales.
AstraZeneca's Tagrisso was approved by the MFDS in May 2016 for "local progressive or metastatic non-small cell lung cancer patients with EGFR-T790M mutations confirmed after EGFR-TKI administration." It is prescribed as a secondary treatment for non-small cell lung cancer patients who have developed resistance after administration of the existing first and second generations of EGFR-TKI.
Tagrisso's sales soared in December 2017 when it was covered by health insurance benefits as a secondary treatment.
Tagrisso's sales, which started at ₩2.3 billion in the first year of release and stayed at ₩10.3 billion in 2017, jumped 5.8 times to ₩59.4 billion in 2018.
Although it continued to grow rapidly with ₩79.2 billion in 2019 and ₩106.5 billion in 2020, it is not as good as it used to be recently.
After setting its own record of ₩27.9 billion in sales in third quarter of last year, it continued to decline for two consecutive quarters.
Tagrisso was added to the primary therapy indication in Korea in December 2018, and the reimbursement process has been stalled for more than two years.
Tagrisso, which is not covered by insurance, costs ₩217,782 per 80mg, more than ₩6 million a month.
Even if it meets the standards, there are many patients who cannot be treated because of the cost burden.The release of generics also seems to have had some impact.
Since last year, global clinical subjects have been actively recruiting third-generation EGFR-TKI Leclaza (Lazertinib) therapy developed by Yuhan.
Pfizer's Vizimpro (Dacomitinib), a small but second-generation drug, also began to be prescribed late last year after being listed on the benefit.
Sales of Giotrif in the first quarter of last year were ₩5.2 billion, up 10.1% year-on-year.
This is its biggest sales since its launch in South Korea.
The remaining EGFR-TKI sales are sluggish.
First quarter sale of Iressa by AZ were ₩4.2 billion, down 29.9% from a year earlier.
During the same period, Roche's Tarceva recorded ₩1.5 billion in sales, down 29.6%.
Vizimpro's sales in fourth quarter of last year and first quarter of this year are still insignificant.
Industries are predicting that changes in EGFR-TKI markets will also accelerate during second half of this year.
It is observed that competition between the 3rd generation EGFR-TKI will intensify after the launch of the domestic new drug Leclaza (Lazertinib).
Leclaza was approved by the MFDS in January as a treatment for patients with EGFR T790M mutations that had previously been treated with EGFR-TKI.
The HIRA's Cancer Drugs Benefit Application Committee, which was held in February, has been properly assessed and negotiated between the Pharmaceutical Benefits Advisory Committee and the NHIS.
It is predicted that sales will begin in earnest from the second half of this year after completing the registration process quickly.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









