- LOGIN
- MemberShip
- 2025-12-26 10:40:28
- 3-way race between ultra-expensive new orphan drugs to start
- by | translator Alice Kang | 2021-06-01 06:11:27

The introduction of 3 new drugs in just 3 years marked the start of a full 3-way race between the new SMA treatments in Korea.
In the U.S.
where the race had already started, Spinraza’s share in the market has been decreasing.
As to whether the situation would be the same in Korea is gathering attention.
The Ministry of Food and Drug Safety (MFDS) has approved Novartis’ ‘Zolgensma (onasemnogene abeparvovec-xioi)’ as the second advanced biological product in Korea.
Zolgensma can be used in SMA patients with a double allelic mutation in the SMN1 gene who have been ▲ clinically diagnosed as SMA Type 1 or ▲ has three or fewer copies of the SMN2 gene.
Zolgensma’s different mechanism of action works as a strength over to Spinraza or Evrysdi SMA is a serious rare disease in which muscles gradually degenerate as motor nerves are damaged due to a gene defect in the survival motor neuron 1 (SMN1) gene.
One out of every 10,000 infants around the world are diagnosed with SMA, and in Korea, around 30 patients (per 300,000 infants) are diagnosed with SMA every year.
The severity of SMA is deeply associated with the number of copies of the “backup” SMN2 gene.
The SMN2 gene may produce and compensate at most 10% of the SMN protein unable to be produced by SMN1.
Patients with Type 1 SMA that have only 1-2 SMN2 copy genes may experience 95% loss of their motor neurons, and 90% of the patients die before reaching age 2.
In Korea, there had been no SMA treatment available until 2017.
Biogen’s ‘Spinraza’ was the first to be approved in December 2017, opening up a new treatment paradigm.
It is an antisense oligonucleotide splicing modulator that promotes protein production by binding to the pre-mRNA sequence of the SMN protein produced by SMN2.
After Spinraza, Roche also received approval for its RNA-based treatment, ‘Evrysdi.’ Unlike Spinraza, which is an injection formulation, Evrysdi is an oral formulation and is cheaper.
However, this oral drug needs to be taken every day.
Evrysdi has not yet been released in the Korean market.
Zolgensma’s mechanism of action is completely different from the two drugs that were previously approved.
Unlike Spinraza and Evrysdi, which use the “backup” SMN2 gene to increase the production of proteins, Zogensma completely replaces the function of the missing SMN1 gene to produce SMN proteins.
When the replacement, made with a recombinant AAV adeno-associated virus (AAV9), is given to an infant by intravenous infusion, it works as an SMN1 gene and produces proteins.
It is also called a 'dream cure' as patients can expect to be completely cured with just a single treatment.
As a “one-shot treatment,’ the cost of the drug is also ultra-expensive.
The cost of a single Zolgensma infusion costs 2.5 billion won.
This is 25 times higher than the cost of Spinraza.
However, Novartis explains that the cost-effectiveness of Zolgensma is quite high considering that Spinraza or Evrysdi would cost 0.3-0.5 billion won every year.
Spinraza’s sales falter and Evrysdi strong in the U.S….
how about Korea? The SMA market in Korea is currently dominated by Spinraza.
Based on IQVIA, Spinraza sold 72 billion won in sales last year.
However, with the entry of Zolgensma and the yet-to-be-introduced Evrysdi, the market dominion in Korea is expected to change.
Changes in the U.S.
SMA market that is already in the 3-way race, can be used as a reference to predict the future of the Korean market.
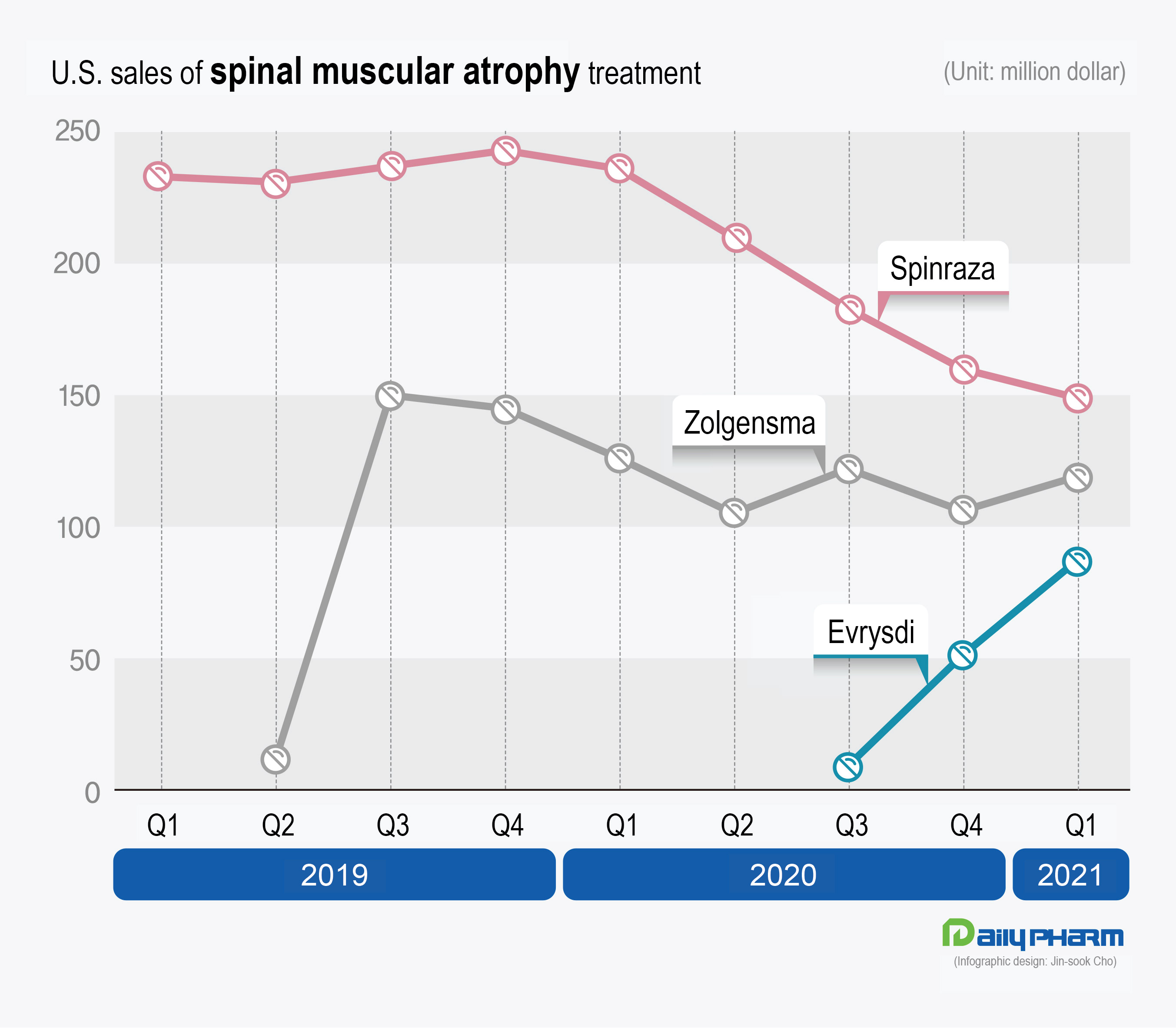
In the U.S.
Spinraza’s sales have been showing a decline with the introduction of Zolgensma and Evrysdi.
After selling 233 million dollars (approx.
257.3 billion Korean won) in Q1 2019, its sales had started falling from Q2 2020.
The sales fell to under 200 million dollars in Q3 2020 to record 183 million dollars (approx.
202.1 billion won), then to 149 million dollars (approx.
164.5 billion won) in Q1 last year.
This was a 36% decline in sales over the past 2 years.
On the other hand, Zolgensma’s sales have shown a similar record every quarter since its introduction, since Zolgensma is a one-shot treatment that does not accumulate patients.
After recording 150 million dollars (165.6 billion won) in Q3 2019 when it started to be prescribed in earnest, its sales have stayed steady in the 100 million to 130 million dollar range (143.5 billion won) every quarter.
Evrysdi, which was introduced last in the U.S.
market, has been relatively rapidly gaining its share compared to the other two competitors.
After receiving approval in August 2020, Evrysdi sold 9 million dollars (9.9 billion won) in Q3 of the same year, which increased to 51 million dollars (56.3 billion won) in Q4, then to 87 million dollars (96.1 billion won) in Q1 this year.
It may have had the lowest sales record, but its sales growth is the fastest among the three products.
Based on the trend, U.S.
experts expect Evrysdi to record the highest sales in 2026 based on its low-cost and oral formulation.
Of course, the U.S.’s case may not apply 100% to Korea, as the Korean market is most strongly influenced by whether the drug is reimbursed as well as the reimbursement criteria.
However, Spinraza’s sole lead in the Korean market is expected to continue for the time being, until reimbursement is approved for Evrysdi and Zolgensma.
Also, there is criticism that the current insurance policy structure makes it difficult to accommodate ultra-expensive drugs like Zolgensma.
The various clinical trials ongoing with Spinraza are also a variable.
Spinraza has been conducting various clinical trials to maintain its lead in the SMA market; one of which is on asymptomatic infants.
This study investigates whether administering Spinraza in advance to infants that have been genetically diagnosed with SMA helps to maintain a normal level of motor function in the patients.
If Spinraza is approved for the indication to treat infants with confirmed SMN1 gene defect or mutation prior to diagnosis, Spinraza will be available at the very front-end, before any other treatment.
In addition, Biogen has started more aggressive trials as well.
The Phase IV RESPOND trial studies the benefit of switching to Spinraza in patients who showed a suboptimal clinical response to Zolgensma.
Biogen identified cases of some patients who received Zolgensma but were insufficiently treated and found that switching to Spinraza may bring additional benefits to these patients.
A long-term follow-up study showed that 4 out of 10 patients that had previously received Zolgensma have switched to Spinraza to continue their treatment.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









