- LOGIN
- MemberShip
- 2025-12-25 20:53:37
- Domestic Rx sales are showing strength in H1
- by An, Kyung-Jin | translator Choi HeeYoung | 2021-07-21 05:51:17

Drugs developed with domestic technologies such as "Rosuzet" and "Zemimet" led by HK-Inoen's "K-CAB" have increased their market influence despite the chaos of COVID-19 outbreak.
The originals of multinational pharmaceutical companies, which once had sales, have stagnated since the expiration of patents.
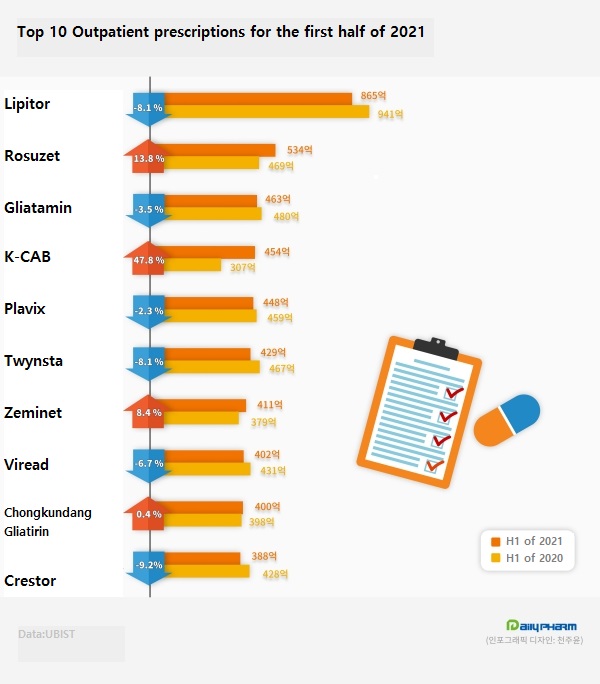
According to UBIST, a pharmaceutical research agency on the 19th, Pfizer's Lipitor topped the list with a cumulative prescription amount of ₩86.5 billion in the first half of this year.
Lipitor (Atorvastatin) is a treatment for dyslipidemia, which was introduced to the domestic market in 1999 by Pfizer Korea.
Since the expiration of the patent, insurance drug prices have fallen by half and more than 130 generics have been released, but they are still showing strong sales in the prescription drug market.
Except for 2017, it has topped the prescription list for the past 10 years.
Viatris, which was launched in November last year with the merger of Upjohn and Mylan, which have been in charge of Pfizer's patent-expired drugs, is in charge of sales.
However, looking at the quarterly performance, the upward trend has slowed somewhat since COVID-19 outbreak began in earnest.
By 2019, the average amount of Lipitor prescription per quarter was close to ₩48 billion, but the average of last year was barely over ₩46 billion.
This year's performance was ₩43 billion in the first quarter and ₩43.5 billion in the second quarter, which is not up to its peak performance.
The cumulative amount of prescriptions for the first half of the year decreased by 8.1% from ₩94.1 billion a year earlier.
The prolonged COVID-19 situation is believed to have been affected by the contraction of hospital visits and the overall foreign prescription market.
During the same period, Hanmi's Rosuzet ranked second in prescriptions, with outpatient prescriptions rising 13.8% year-on-year to ₩53.4 billion.
Rosuzet is a combination drug composed of Ezetimibe and Rosuvastatin.
Since its release in late 2015, it has ranked first in the market among the same ingredients.
Analysts say that Hanmi's strategy to enter the composite market before its competitors was effective as it secured Ezetimibe license from patent holder MSD.
Sales of Rosuzet rose more than 10% year-on-year every month even at a time when face-to-face sales marketing activities were severely restricted due to COVID-19 last year.
In the second half of last year, the monthly prescription amount exceeded ₩8 billion, surpassing Gliatamin as the second-largest outpatient prescription.
This year, the company set its own record of ₩9.4 billion in monthly prescription amount, narrowing the gap with No.
1 Lipitor.
Sales of Daewoong Bio's Gliatamin was ₩46.3 billion in outpatients in the first half of last year, down 3.5% from a year earlier.
Gliatamin is generic of the brain functional enhancer Choline alfoscerate.
The government has received two sanctions, including a reduction in benefit.
However, Gliatamin's influence in the prescription drug market remains robust.
The competition item, Chongkundang Gliatirin, increased 0.4% year-on-year to ₩40 billion in outpatient prescriptions over the same period.
The amount of outpatients for K-CAB in the first half of the year increased 47.8% year-on-year to ₩45.4 billion.
If the current trend continues, it is predicted that the amount of outpatients will easily exceed ₩100 billion by the end of the year.
K-CAB (Tegoprazan) is a flagship product of HK Inno.N (formerly CJ Healthcare), which is about to be listed.
It is a new type of anti- ulcer drug called "P-CAB," which competitively combines proton pumps and potassium ions located in the final stage of acid secretion in stomach wall cells, thus inhibiting gastric acid secretion.
Sales of K-CAB rose to ₩5 billion in the first year of its release, compared to ₩1.7 billion in monthly prescriptions.
It secured gastrointestinal reflux disease as its first indication, and added gastrointestinal ulcer treatment in July of the same year.
In the first half of this year, when most of the drug performance was sluggish, it rose to fourth place in prescription, threatening third-place Gliatamin.
The joint sales strategy with Chong Kun-dang, which has a differentiated mechanism from the existing PPI.
The increase in prescriptions for products developed by domestic companies was noticeable.
The amount of outpatients for LG Chem's diabetes combination drug Zemimet in the first half of this year was ₩41.1 billion, up 8.4% from the previous year.
Zemimet is a second compound that combines Metformin with Zemiglo (Gemigliptin), a new diabetes drug developed by LG Chem.
It has been co-selling with Daewoong Pharmaceutical since 2016.
Due to changes in the diabetes treatment market, which prefers multi-drug prescriptions, it has surpassed the performance of Zemimet.
In the top 10 outpatient Rxs, sales of prescriptions for the past six months have fallen.
The amount of outpatient prescription for Sanofi's anti-thrombotic drug Plavix was ₩44.8 billion, down 2.3% from the previous year.
Boehringer Ingelheim's hypertension combination drug Twynsta and Gilead Science's hepatitis B treatment Viread fell 8.1% and 6.7% in the first half, respectively.
AstraZeneca's treatment for dyslipidemia, Crestor, decreased 9.2% from the previous year to ₩38.8 billion in the first half of the year, ranking among the top 10 outpatients Rx.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









