- LOGIN
- MemberShip
- 2025-12-25 20:53:37
- 19% of valsartan Rxs disappear 3 years after impurity issue
- by An, Kyung-Jin | translator Alice Kang | 2021-07-27 05:35:36
Three years have passed since the valsartan impurity issue swept across the pharmaceutical industry, however, the valsartan market is still showing no signs of recovery.
Prescriptions of products containing valsartan as monotherapy and combination therapy both decreased by nearly 20%.
Analysts believe that distrust in valsartan after the impurity issue has led to switching prescriptions to similar drugs.
According to the pharmaceutical research institution UBIST on the 26th, outpatient prescriptions for valsartan monotherapy products recorded ₩29.9 billion in the first half of this year, a 5.4% decrease from the same period of the previous year.
This is the second consecutive year of decline since the 4.1% decrease to ₩31.6 billion last year.
Compared to the ₩34.1 billion in 1H of 2018, prescription of valsartan monotherapy products decreased by 12.4% in three years.
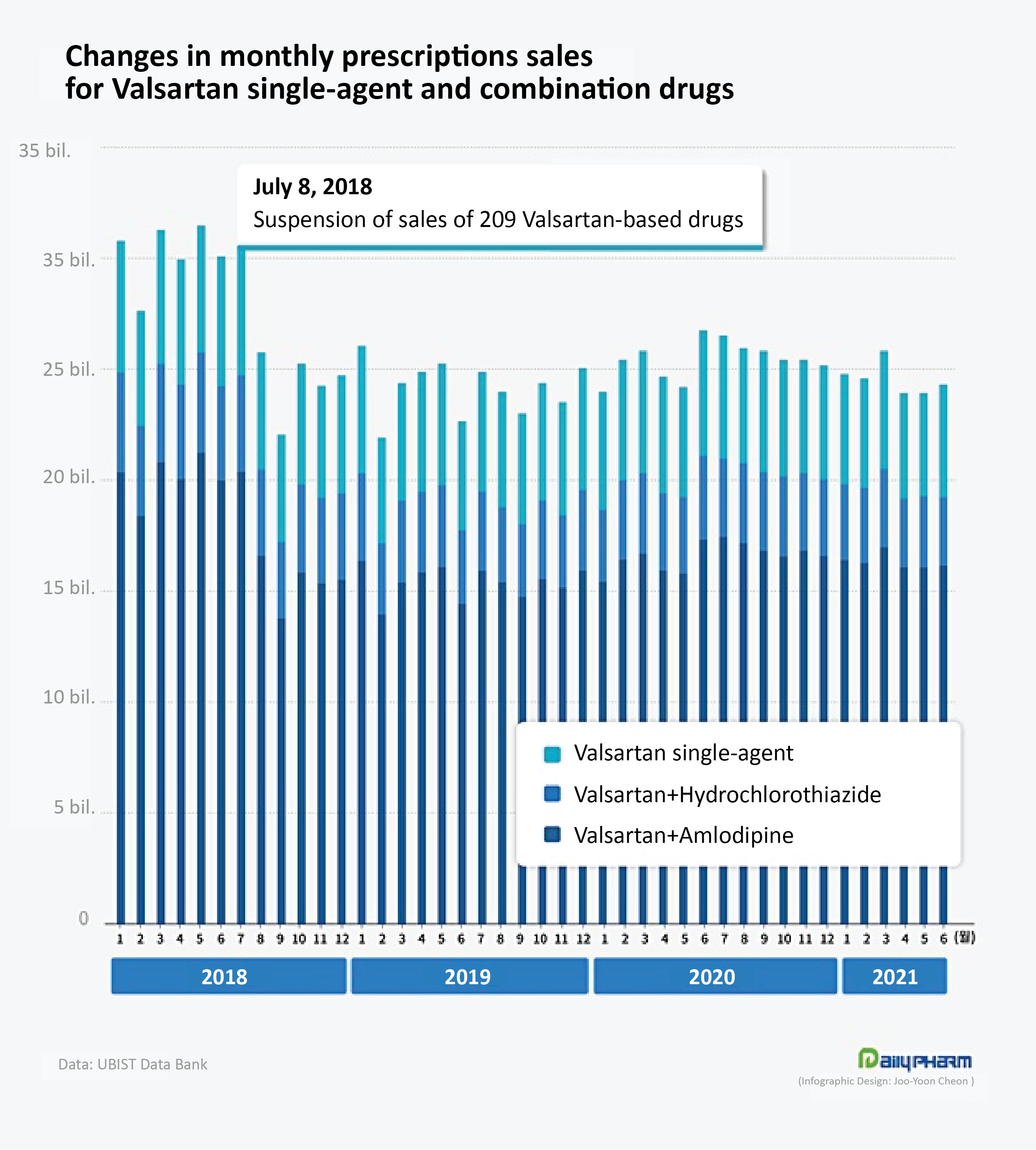
The valsartan market had contracted much since an N-Nitrosodimethylamine (NDMA) impurity was detected in the valsartan active ingredient from China's Zhejiang Huahai Pharamceutical, which led to unprecedented mass recalls and sales suspensions around the world.
This incident had delivered a hard blow to the growing domestic prescription market for valsartan products.
On July 8th, 2018, the Ministry of Food and Drug Safety had suspended the sale of 209 pharmaceutical products containing valsartan that were being distributed in Korea for using an active ingredient that was found with NDMA.
Afterward, the authorities made remedies for products that did not use the active ingredient in question and additionally uncovered products that use NDMA containing active ingredients, resulting in a total of 175 products being suspended from sales.
The valsartan combination prescription market, which had a larger market than the monotherapy market, was also unable to escape the aftermath of the impurity crisis.
In the first half of this year, the prescription volume for valsartan in combination with a calcium channel blocker (CCB) class amlodipine amounted to ₩98.1 billion.
This was a 19.0% decline from the ₩121.1 billion in the first half of 2018, just before the impurity was detected.
The market had somewhat recovered – by 6.6% - compared to the first half of 2019 when the valsartan issue just hit the industry.
The combination of valsartan and the diuretic hydrochlorothiazide sold ₩19.3 billion in prescriptions in the first half of this year.
From the ₩25.5 billion that was recorded in 2018 to ₩21.5 billion in 2019, and to ₩21.1 billion in 2020, the record has continued to fall.
Compared to 3 years ago, prescriptions of the valsartan and hydrochlorothiazide combination fell 24.5%.
Combined, the monotherapy and combination therapy market for products containing valsartan recorded ₩147.3 billion in the first half of this year.
This was an 18.5% decrease from the ₩180.8 billion recorded in 2018.
Until the first half of 2018 before the impurity issue broke, the monthly prescription of valsartan monotherapy and combination therapy products exceeded ₩30 billion per month.
The monotherapy market by itself had recorded ₩6 billion in prescriptions every month.
However, the prescription of valsartan monotherapy and combination therapies that reached ₩30.5 billion in July of 2018, fell immediately after the impurity issue, falling sharply to ₩25.8 billion in August, and ₩22 billion in September.
And for three years since then, the monthly prescription has never exceeded ₩30 billion.
As of June this year, the prescription for valsartan monotherapy and combination therapy combined recorded ₩24.3 billion.
In other words, one-fifth of the prescriptions for valsartan disappeared after the impurity issue.
Analysts believe that the distrust built in valsartan after the impurity issue has led to an avoidance of prescriptions containing valsartan.
It is highly likely that some of the existing valsartan prescriptions have been switched to similar drugs.
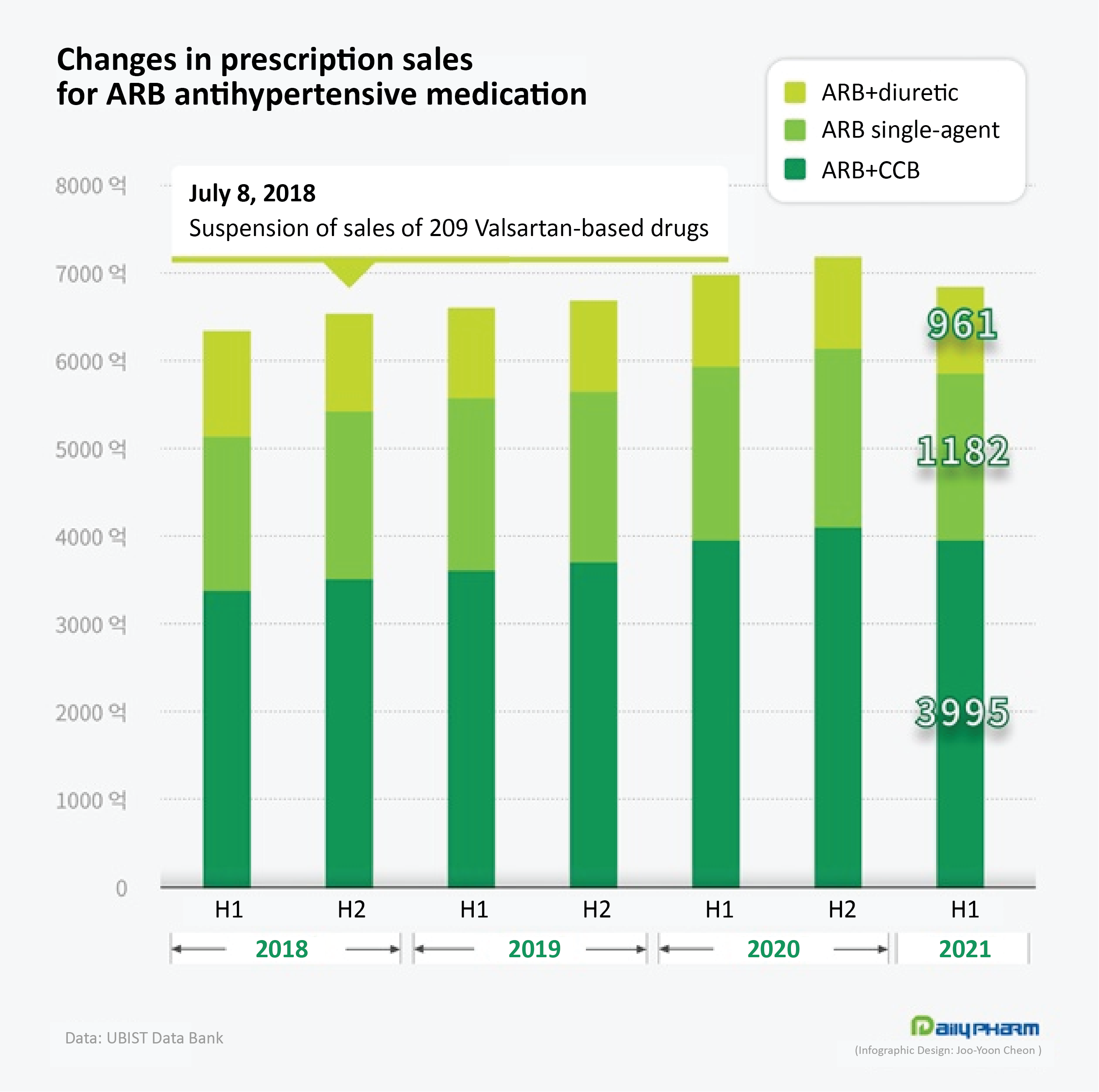
The prescription for ARB-class monotherapies in the first half of this year amounted to ₩188.2 billion.
As the pharmaceutical market had contracted in general due to the prolonged COVID-19 pandemic, the ARB market had also seen a decrease of 5.1% compared to the same period of the previous year but had increased by 2.9% compared to the ₩182.8 billion in 2018.
The dual combination therapy market for the ARB+CCB class of antihypertensive ingredients had grown even more steeply.
The accumulated prescription amount for the ARB+CCB combination in the first half of this year was ₩399.5 billion.
This was an 0.2% increase from the same period last year and an overwhelming 19.1% increase from the ₩335.5 billion in the first half of 2018.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









