- LOGIN
- MemberShip
- 2025-12-25 20:53:38
- Domestic DDP-4 inhibitors fare better amid sales decline
- by Kim, Jin-Gu | translator Alice Kang | 2021-07-28 05:55:04
Prescription of DPP-4 inhibitors, which used to drive the diabetes treatment market, are on the decline.
In just a single year, the market size of DPP-4 inhibitors decreased 4%, and this is the third consecutive quarter the market saw a decline since Q4 last year.
Also, multinational companies and domestic companies have seen opposite results in the sales performance of their products.
While prescriptions of all DPP-4 inhibitors from multinational companies fell, most products from domestic companies continued to make growth.
◆Is the party over for DPP-4 inhibitors?...
market decreased 4% in one year According to the pharmaceutical market research institution UBIST on the 27th, the diabetes drug market size for DPP-4 inhibitors was 141.6 billion won in Q2 this year.
This was a 4% decline compared to the 148.2 billion won recorded in Q2 last year.
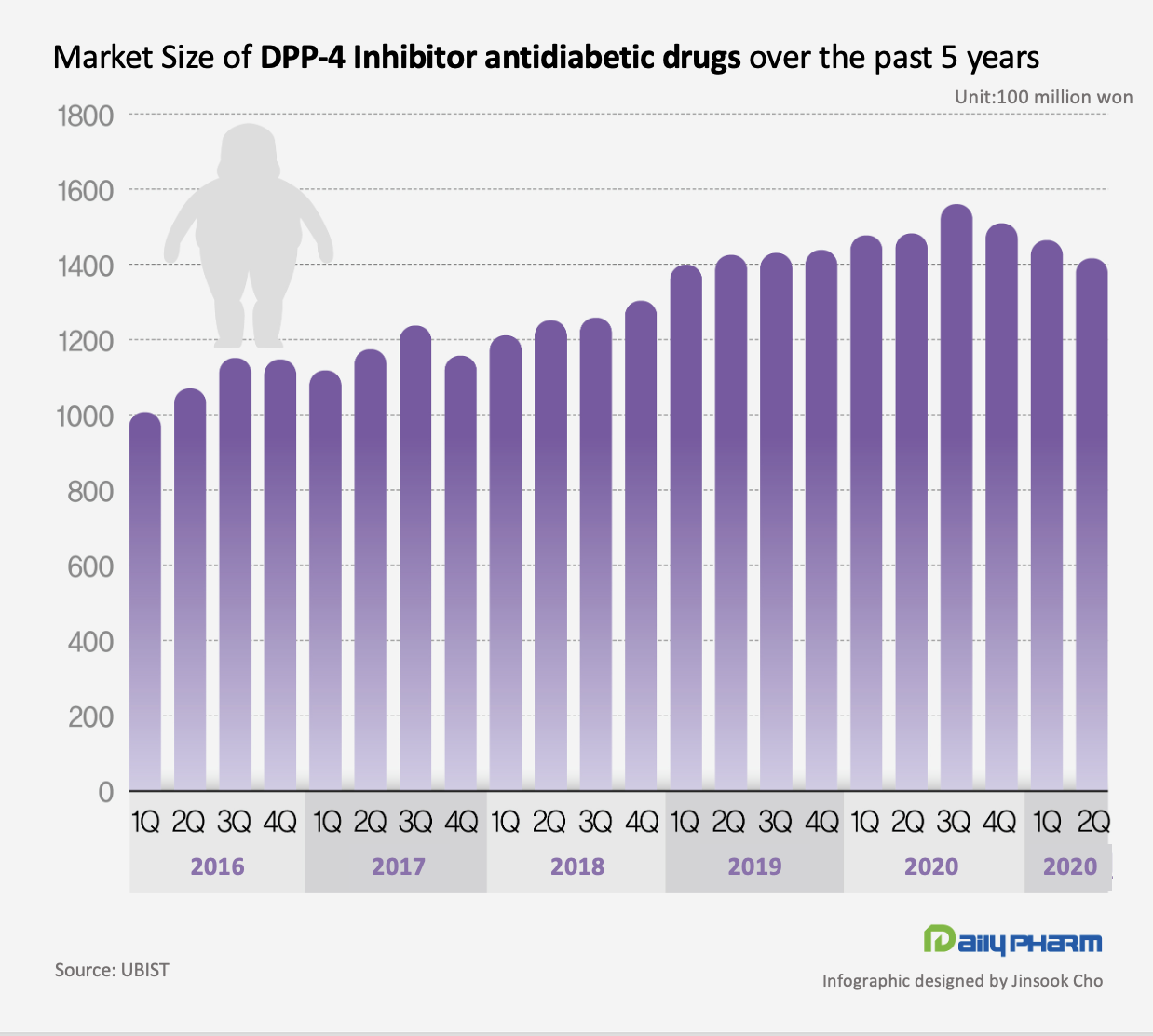
After first being formed in 2008 with the introduction of MSD’s ‘Januvia (sitagliptin),’ the market saw repeated growth with the release of latecomer drugs and became 'the most frequently prescribed diabetes drug.’ In Q1 2016, the market made the first quarterly sales that exceed 100 billion won.
The market peaked in Q3 last year and made 156 billion won.
However, since then, the market saw 3 consecutive quarters of decline, from 156 billion won.
(4Q 2020) to 146.4 billion won (Q1 2021), to 141.6 billion won (Q2 2021).
If this trend persists, the overall market size of antidiabetics is expected to fall below 600 billion won by the end of this year.
◆Market No.1 and 2 – ‘Januvia’ and ‘Trajenta’ both decline 8% Multinational pharmaceutical companies and domestic companies have seen opposite results in the prescriptions of their products.
While prescriptions of products from multinational companies all declined, most products developed or introduced by domestic companies saw a growth in sales.
The market’s leading product, MSD’s Januvia series saw an overall decline of 8% from the 43.4 billion won in Q2 last year to 40.1 billion won in Q2 this year.
The sales drop was more vivid in its single-agent product.
Januvia’s sales fell 11%, while sales of the combination drugs Janumet and Janumet XR decreased 8% and 3%, respectively.
The same went for the market’s runner-up product ‘Trajenta (linagliptin).’ Its sales dropped 8% from 31.9 billion won to 29.3 billion won in the same period.
Both the single-agent and combination drugs Trajenta and Trajenta Duo saw an 8% drop in sales.
Sales of Novartis’ ‘Galvus (vildagliptin)’ series dropped 7% from 10.9 billion won to mark 10.7 billion won.
Sales of Takeda Pharmaceutical's ‘Nesina (alogliptin)’ series fell 11% from 7.9 billion won to 7.1 billion won.
Also, sales of AstraZeneca’s Onglyza (saxagliptin) series also fell 7% from 6.8 billion won to 6.3 billion won.
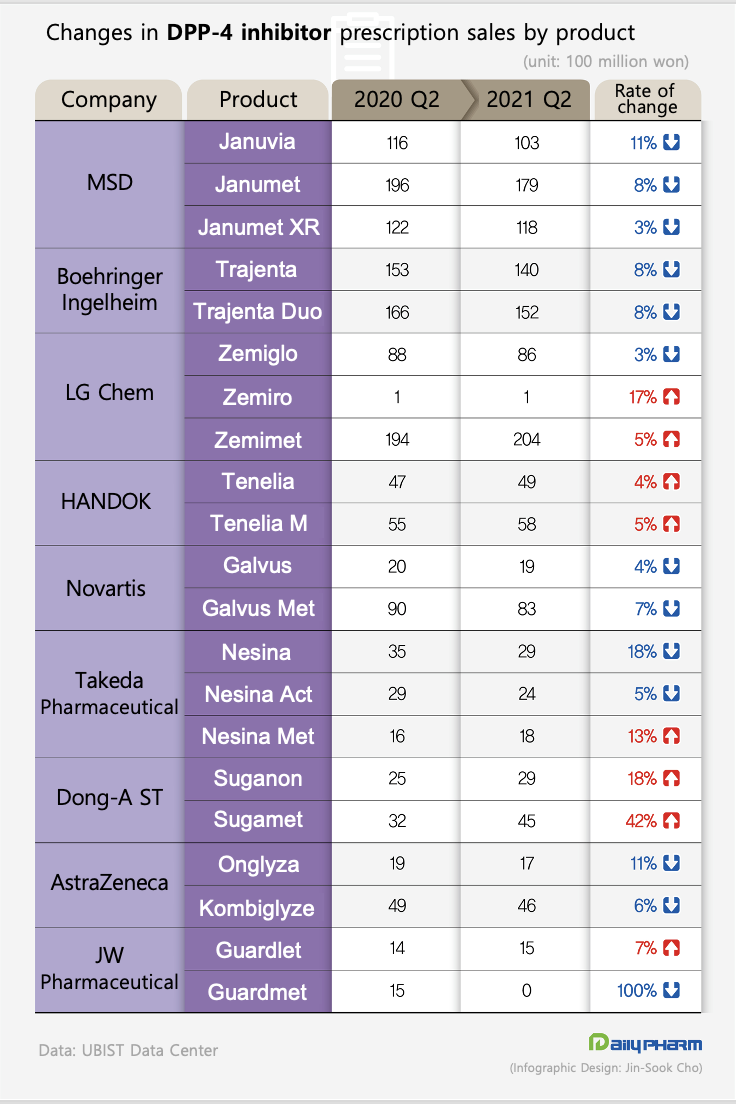
Sales of LG Chem's 'Zemiglo (ingredient name: gemigliptin)' series, which had ranked third in the market, increased by 2% from 28.4 billion won to 29.1 billion won in a single year.
Despite the 3% sales decline of the single-agent Zemiglo (8.8 billion won → 8.6 billion won), the overall prescriptions performance of the series increased with the 5% increase (19.4 billion won → 20.4 billion won) in sales of the combination drug Zemimet.
However, it is evaluated that the overall growth drive has somewhat weakened compared to the past.
Zemiglo and Suganon and self-developed new durgs, Tenelia and Guardlet were introduced to Korea Sales of Handok’s ‘Tenelia (teneligliptin)’ increased 4% from 10.3 billion won to 10.7 billion won in the same period.
Sales of the single-agent drug Tenelia grew 4%, and the combination drug Tenelia M grew 5%, respectively.
Tenelia is a diabetes drug from Japan’s Mitsubishi Tanabe that Handok introduced to Korea.
Using Tenlia, Handok had self-developed the combination drug Tenlia M by adding metformin.
Currently, the combination drug is prescribed more than the single-agent drug.
Dong-A ST’s self-developed new drug ‘Suganon (evogliptin)’ series has been showing the most rapid growth among all drugs recently.
Its prescriptions only amounted to 5.7 billion won in Q2 last year, however, this increased 31% to mark 7.4 billion won in just one year.
The Suganon series, which was introduced in Q2 2016, was the last to enter the DPP-4 inhibitor market.
At that time, its prescriptions amounted to only 0.6 billion won.
However, this had increased over tenfold in just 5 years.
The ‘Guardlet (anagliptin)’ series that JW Pharmaceutical introduced from Sanwa Kagaku Kenkyusho, was the only product to see a decline in prescriptions among all domestic products.
Its prescriptions, which amounted to 2.9 billion won in Q2 last year, decreased to 1.5 billion won.
This decline was influenced by impurities detected in some of the metformin products.
JW Pharmaceutical's Guardmet’s sales were suspended due to the detection of impurities.
Without Gaurdmet, which accounted for over half of the sales in the Guardlet series, prescriptions of the whole family also decreased to around half of what it was before.
However, prescription for the single-agent drug Guardlet itself had increased 7% from 1.4 billion won to 1.5 billion won in one year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









