- LOGIN
- MemberShip
- 2025-12-25 20:53:37
- Sales of PPI antiulcer agents rise 30% in 2 years
- by An, Kyung-Jin | translator Kang, Shin-Kook | 2021-08-05 06:03:05
The ranitidine impurity issue gave wings to the rising prescription of proton pump inhibitors (PPIs).
The share of PPI prescriptions in the antiulcer agent market grew 30% in only 2 years after the drugs containing ranitidine – which used to occupy the largest share of the H2 receptor antagonists - were pulled from the market.
Hanmi’s incrementally modified drug ‘Esomezol’ gained marked influence over the antiulcer drug market with the impurity issue and COVID-19 as momentum.
◆PPI prescriptions increase 5%...reaps benefits despite prolonged COVID-19 crisis According to the pharmaceutical market research institution UBIST on the 4th, outpatient prescription of PPIs recorded ₩616.7 billion in Q2 this year, which is a 5.0% year-on-year increase from the same period of the previous year.
Compared to Q2 2019, sales had risen 29%.
Despite the overall contraction of the prescription drug market due to the prolonged COVID-19 pandemic, PPI prescriptions have maintained quarterly sales of over ₩160 billion during the past year.
The cumulative PPI prescriptions in the first half of this year amounted to ₩323.6 billion, which was a 7.3% year-on-year increase.
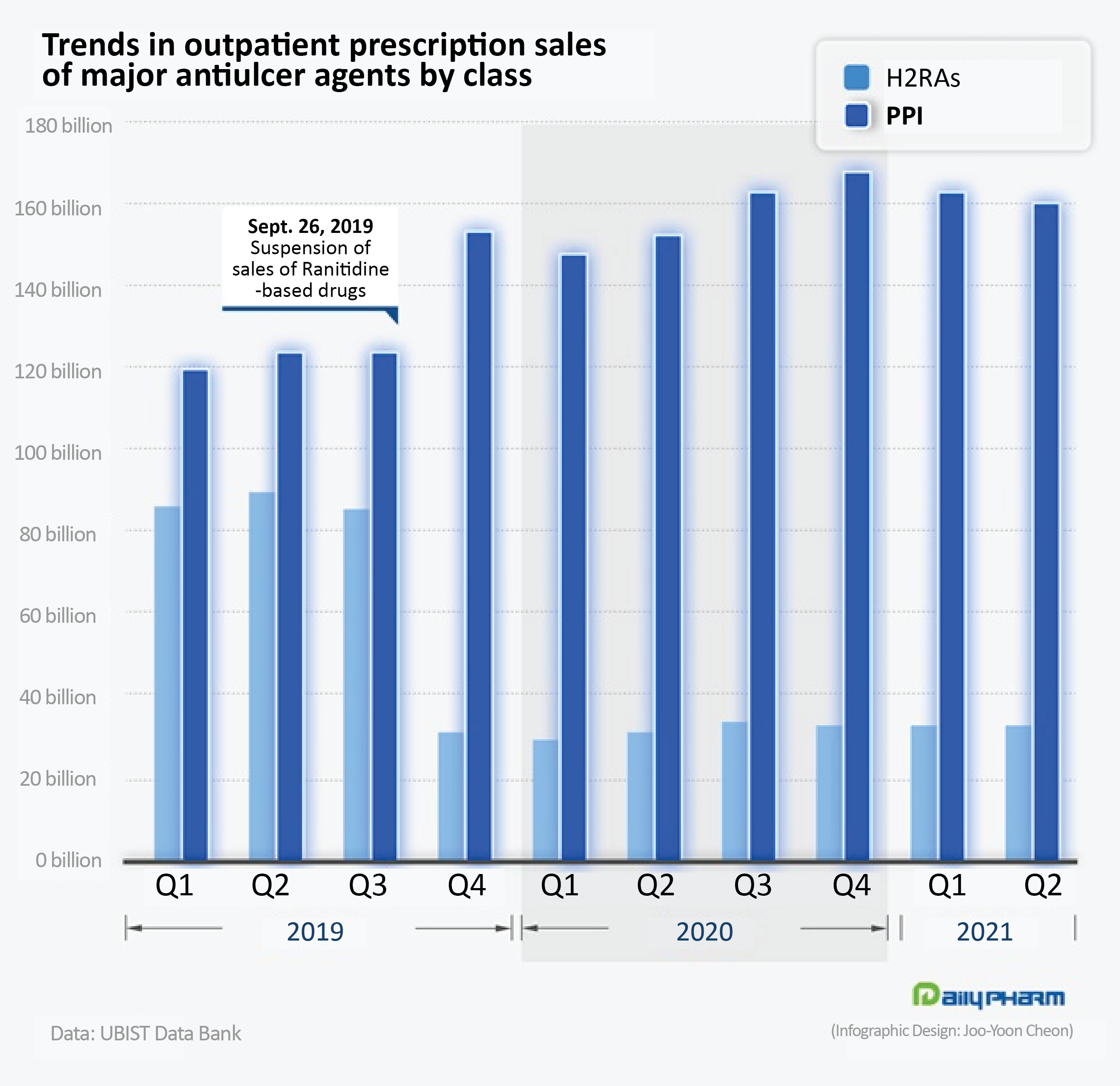
Since recording ₩335.8 billion in outpatient prescription sales in 2015, sales of PPIs have increased over 10% each year to account for the largest share of anti-ulcer drugs prescribed in Korea.
The rate of increase became greater since 2019.
The ranitidine impurity issue was pointed to as the reason for the surge in PPI prescriptions.
Analysts believe the suspended sales of 'ranitidine' products among H2 receptor antagonists that used to hold the largest proportion of prescriptions and had a similar prescription target to PPIs, led to PPIs receiving the benefit.
◆Sales of Esomeprazole jump 33%·rabeprazole 22%...polarization intensifies in PPI prescriptions The ranitidine impurity first led to an improvement in the prescription performance of all the 7 PPI ingredients.
However, after two years, the different PPI ingredients saw mixed results.
Prescription of esomeprazole and rabeprazole, which accounted for a large proportion of prescriptions, have continued to rise after their prescription performance surged in Q4 2019, while the upsurge in the rest of the ingredients was only temporary, showing the intensifying gap between the ingredients.
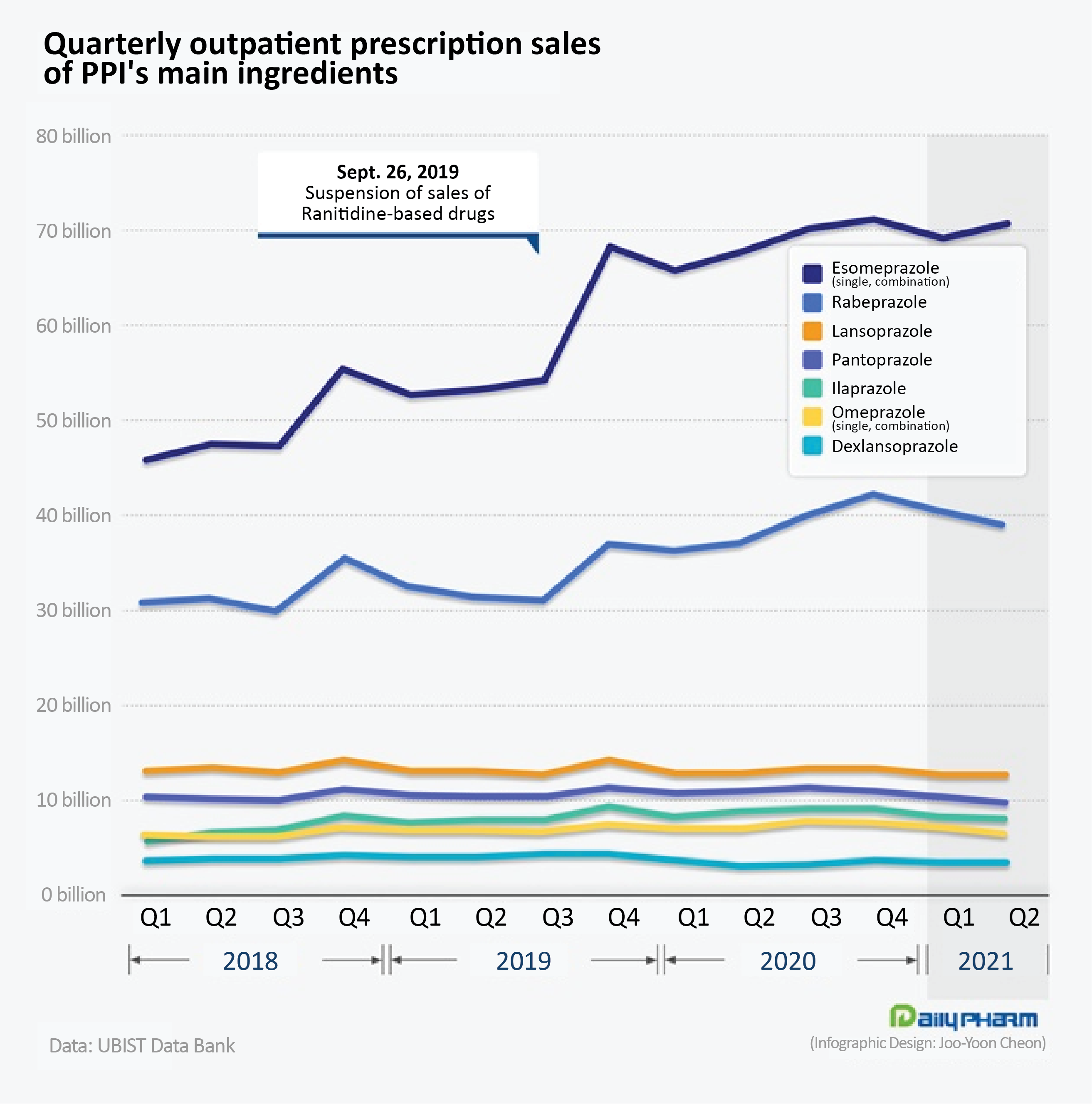
Outpatient prescriptions of esomeprazole amounted to ₩70.4 billion in Q2.
This is a 4.0% increase from the previous year and a 33.3% increase from the year before the last.
In Q3 2019, esomeprazole sold around ₩53.7 billion, however, after ranitidine was removed from the market in Q4 2019, its sales rose sharply to reach ₩67.9 billion.
The upward growth continued afterward to reach ₩70.8 billion in Q4 last year.
The rabeprazole ingredient sold ₩38.1 billion in Q2, marking a 4.5% year-on-year increase.
Rabeprazole’s sales in Q4 2019 rose to ₩36.5 billion and continued its rise to reach ₩ 41 billion in Q4 last year.
Compared to 2Q 2019, prescriptions rose 22.0% in just 2 years.
Although the increase was not as prominent as in esomeprazole or rabeprazole, Ilaprazole also benefited from the market withdrawal of ranitidine.
Ilaprazole’s outpatient prescriptions reached ₩8 billion in Q2.
This was an 8.8% year-on-year decrease, but a 2.3% increase from 2 years before.
The other PPI ingredients received less impact from the impurity issue.
Omeprazole sold ₩6.4 billion in Q2.
This was a 10.2% and 6.6% decrease from the same period a year and 2 years ago.
In the same period, pantoprazole sold ₩9.6 billion, an 11.2% and 6.5% decrease from the previous year and 2 years ago.
Outpatient prescriptions of lansoprazole in Q2 was ₩3.9 billion.
This was an 11.6% year-on-year increase, but a 12.2% decrease from the 2 years ago, showing less performance than usual.
◆Prescriptions of Hanmi’s 'Esomezol' rise 45%...Products see mixed results from COVID-19 The key PPI products have experienced a sharp change in their prescription performance during the past 2 years, with the lessening impact of the impurity crisis and the increasing impact of the COVID-19 crisis.
Hanmi Pharmaceutical’s ‘Esomezol’ showed the most marked growth.
In 1H this year, Esomezol sold ₩23.3 billion in outpatient prescriptions.
Despite the COVID-19 crisis, prescription of the drug increased 14.2% year-on-year to exceed ‘Nexium’ and become the leader in the PPI prescription market.
Esomezol is a salt-modified drug of esomeprazole.
The impurity issue led to an upsurge in prescription demand for Esomezol, with outpatient prescriptions rising 44.8% in just 2 years.
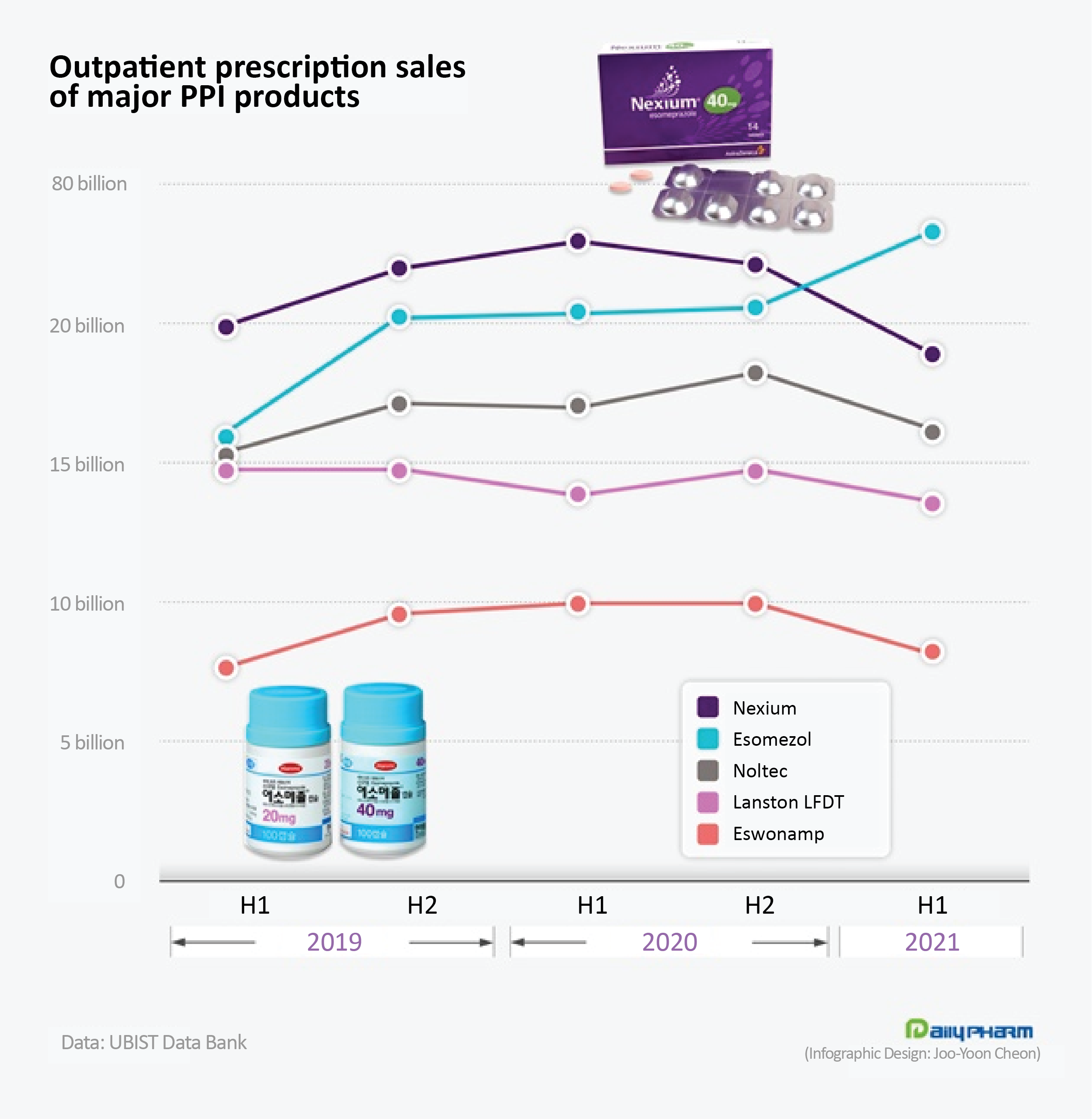
This is a 17.6% decrease from the year before and a 5.8% decrease from 2 years ago.
Due to the COVID-19 crisis, prescriptions of Nexium decreased significantly, enough to hand over its long-held lead in the PPI prescription market.
Nexium is the original esomeprazole brand that is distributed and sold by Daewoong Pharmaceutical in Korea.
The other items were also not free from the impact of COVID-19.
Il-Yang Pharmaceutical’s ‘Noltec’ sold ₩16.2 billion in 1H this year.
This was a 4.3% year-on-year decrease, but also a 4.7% increase from 2 years ago.
Noltec was able to maintain its 3rd place among prescriptions of single-agent PPIs.
Noltec, an ilaprazole PPI agent developed by Il-Yang Pharmaceutical, was released as the nation’s 14th new indigenous drug at the end of 2009.
Takeda Pharmaceuticals Korea’s ‘Lanston LFDT’ also rose to the ranks in H1 this year, selling ₩13.8 billion in outpatient prescriptions.
In the same period, Daewon Pharm's ‘Eswonamp’ sold ₩8.3 billion.
After the ranitidine issue, Eswonamp had enjoyed a profit with the annual prescription amount exceeding ₩20 billion for the first time, but prescription performance fell 17.3% this year.
In addition to the impurity issue, analysts believe that the COVID-19 pandemic has acted as a variable that increased the gap between prescriptions of the products.
HK Inno.N’s release of the antiulcer agent ‘K-Cab (tegoprazan) that has a new mechanism of action, has also intensified the change in the dominion of antiulcer drug prescriptions.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









