- LOGIN
- MemberShip
- 2025-12-25 20:56:14
- Accumulated exports of K-Biosimilars reach ₩9Tril
- by Chon, Seung-Hyun | translator Alice Kang | 2021-08-23 05:55:26
Cumulative exports of biosimilars developed by Celltrion and Samsung Bioepis exceeded ₩9 trillion.
Growth has slowed compared to the products’ first market entry, however, the products have been marking stable growth based on their solid pipeline.
◆Celltrion Healthcare exports ₩789.5billion in H1…cumulative sales exceed ₩6 trillion According to the Financial Supervisory Service on the 19th, Celltrion Healthcare’s exports reached ₩789.5 billion, a 1.6% increase from the ₩777.2 billion in the same period last year.
Although growth was not as explosive as in the previous year, the company still recorded large-scale overseas exports.
Celltrion Healthcare is an affiliate of Celltrion that has Celltrion Healthcare Holdings (share 24.3%) as its largest shareholder.
Celltrion Healthcare sells antibody biosimilar products that it received from Celltrion to global distributors.
In other words, Celltrion Healthcare’s sales refer to the export performance of biosimilars developed by Celltrion.

Remsima’s original is Janssen’s ‘Remicade.’ Remsima SC is a subcutaneous injection formulating of Remsima.
Truxima and Herzuma are biosimilars of ‘MabThera’ and ‘Herceptin,’ respectively.
By each product, Remsima sold the most in exports, recording ₩302.1 billion in H1 this year.
Adding Remsima SC(₩35.1), the Remsima brand sold ₩337.3 billion in exports in H1 this year.
Remsima is the first biosimilar product that was approved in 2012.
It had annually recorded the highest export sales among all Celltrion’s biosimilars but was outrun by Truxima’s record of ₩786.8 billion last year.
However, this year, sales of Remsima again surpassed that of Truxima and became the No.1 product in exports.
Truxima’s export sales fell 25.3% YOY from ₩365.8 billion to ₩273.3 billion in H1 this year.
The company explained that Truxima’s sales in Q1, which saw a 32.6% YOY decrease to mark ₩109.7 billion, was due to a temporary adjustment in the supply schedule of its products.
Herzuma’s export sales in H1 this year were ₩102.3 billion, a 25.7% YOY increase.
Herzuma’s share in the European market in Q1 was 15%, a slight decrease from the 19% it held in Q1 last year.
However, in the Japanese market, its share exceeded that of its originator Herceptin, to record 50%.
This was more than a twofold market expansion in a single year from the 25% share it held in Q1 last year.
Celltrion Healthcare, which was listed on KOSDAQ in 2017, has been recording its export performance in its business report since 2014.
Since 2014, Remsima and Remsima SC have recorded the most in exports with a cumulative record of ₩3.7123 trillion.
Truxima, which started making export performance in 2017, marked ₩1.9925 trillion, and Herzuma’s cumulative exports amounted to ₩575.1 billion.
On whole, Celltrion Healthcare’s cumulative exports from 2014 to H1 this year marked ₩6.3 trillion.
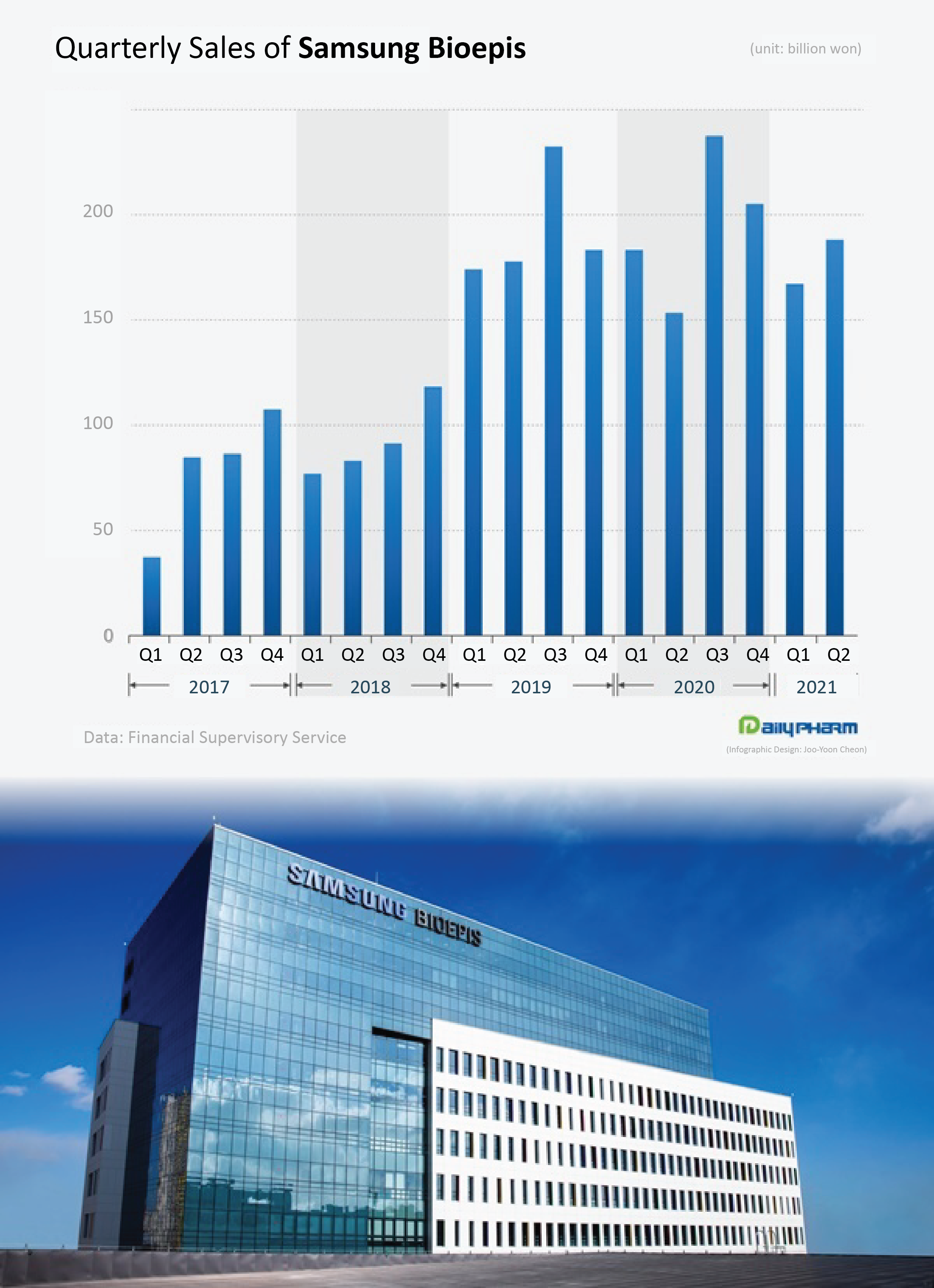
◆Samsung Bioepis makes ₩354.2 billion in H1… cumulative sales record ₩2.0889 trillion Samsung Bioepis’s operating profit last year was ₩145 billion, an 18.1% YOY increase from the same period of the previous year.
Since it recorded its first profit in 2019 with ₩122.8 billion, its profit has grown even more in a single year.
Samsung Bioepis recorded ₩187.5 billion in sales in Q2, which is a 22.7% YOY increase.
The company had continued on a downward roll since Q3 of last year, when it recorded its all-time high with ₩236.9 billion, however, in only 2 quarters since then, the company has recovered its performance to a level similar to its pre-COVID-19 performance.
The accumulated sales of Samsung Bioepis in the 1H of this year rose 5.5% compared to the same period of last year, to record ₩354.2 billion Most of its sales were generated from overseas sales of the company’s self-developed biosimilars.
Samsung Bioepis had succeeded in commercializing 5 biosimilars of biopharmaceuticals – ‘Enbrel,’ ‘Remicade,’ ‘Herceptin,’ ‘Humira,’ and ‘Avastin.’
Biogen is in charge of the local distribution and sales of the three autoimmune treatments, ‘ Benepali,’ ‘Flixabi,’ and ‘Imraldi.’ Organon (formerly known as MSD) is selling the three products in countries other than the U.S., Korea, and China, as ‘Renflexis (Remicade biosimilar), ‘Brenzys (Enbrel biosimilar),’ ‘Hadlima (Humira biosimilar).’ Organon is also in charge of selling the two anticancer drugs - the Herceptin biosimilar ‘Ontruzant’ and Avastin biosimilar ‘Avincio’ – abroad.
According to performance reports by Biogen and Organon, biosimilars of Samsung Bioepis had made $573 million (approximately ₩675 billion) in H1 this year.
Sales made by Biogen rose 4.3% YOY to record $407 million (approximately ₩470 billion).
Sales made with biosimilars through Organon were $166 million (approximately ₩195 billion).
Although the drugs sold less in comparison to its performance in the European market, its growth of 43.4% YoY has been received positively due to its growth potential.
Due to the preorders that were made in Q1, sales in Q2 had decreased, then rose sharply in the second half of the year with the technology fee (milestone payments) from signing biosimilar license agreements with Chinese companies such as S Bio and C-Bridge Capital in 2019 being reflected in the revenue.
This year, sales have stabilized to record ₩166.7 billion in Q1 and ₩187.5 billion in Q2.
Samsung Bioepis, which was established in 2012, had shown full growth since recording ₩147.5 billion in sales in 2016.
Last year, sales increased over fivefold compared to four years ago.
Since its launch in 2012, Samsung Bioepis has recorded a cumulative sales of ₩ 2.8895 trillion.
Most of Samsung Bioepis' sales come from overseas sales of biosimilars or technology fees.
Its domestic sales contributions are small compared to exports.
In other words, Celltrion and Samsung Bioepis’ combined exports of biosimilars recorded over ₩ 9 trillion in total.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









