- LOGIN
- MemberShip
- 2025-12-25 20:53:39
- MSD’s Keytruda becomes the 'symbol' of cancer immunotherapy
- by Eo, Yun-Ho | translator Alice Kang | 2021-08-25 05:57:35


of Keytruda “Reinforcing the body’s immunity to attack cancer cells” By some, the idea was regarded improbable.
Not many were convinced that immunotherapy would rise to the position it is in now.
Even without reference to the famous story of how the former US president Jimmy Carter cured melanoma with immunotherapy, cancer immunotherapy has become an important pillar in the management of cancer disease nowadays.
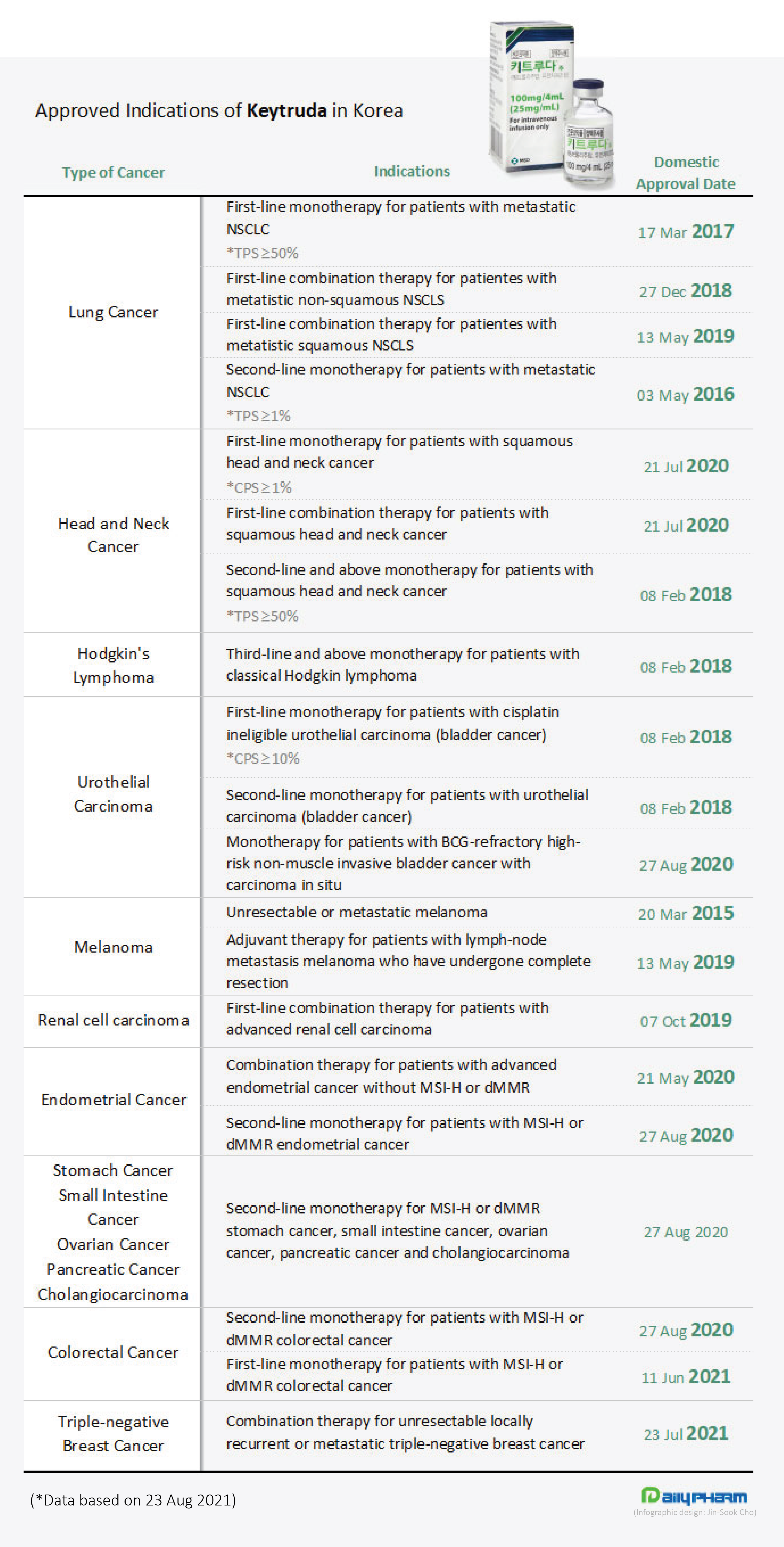
Among the many drugs introduced, MSD’s (Merck in the US) ‘Keytruda (pembrolizumab)’ has risen to become the symbol of ‘cancer immunotherapy.’ The drug, which was first approved in March 2015 as a treatment for melanoma, is currently approved for 18 indications in 14 cancer types in Korea.
One thing to note is that Keytruda is yet far from reaching its turning point.
◆A neglected substance becomes an all-around anticancer drug Keytruda’s history goes all the way back to 2003.
Keytruda was developed as a humanized antibody by Organon, a Dutch pharmaceutical Company.
In 2007, Organon was acquired by Schering Plough, and the substance was approved for clinical trials by the FDA in December 2012.
In 2014, only 3 years after trial approval, the company submitted a New Drug Application to the FDA and received approval as a treatment for melanoma in September of the same year.
This was how Keytruda’s journey began.
One interesting aspect to note is that MSD was not a prominent player in the field of oncology.
The company, whose key areas focused on chronic disease, women’s disease, and vaccines, realized the potential of Keytruda and established a business division for the single drug.
MSD’s insight and drive in making the decision to invest in a substance that was neglected by the initial developer is also one key strength of the company to note.
After the initial melanoma indication, Keytruda was approved as a second-line treatment for non-small cell lung cancer in 2016, an area in fierce competition.
Then, the drug became the first among cancer immunotherapies to receive approval as a first-line treatment for Stage 4 metastatic NSCLC.
Afterward, by adding the combination therapy option to its treatment regiment, the drug’s indication was expanded from patients with high PD-L1 expression to all patients to position itself as the standard treatment option for all patients with metastatic NSCLC.
Also, Keytruda transformed the concept of cancer treatment by becoming the first to be approved for ‘tumor agnostic' use in all cancer types based on specific genetic features regardless of the initial cancer site.
In Korea, the drug was approved as a second-line treatment for 7 types of microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors, and more recently, as a first-line treatment for metastatic MSI-H/dMMR colorectal cancer.
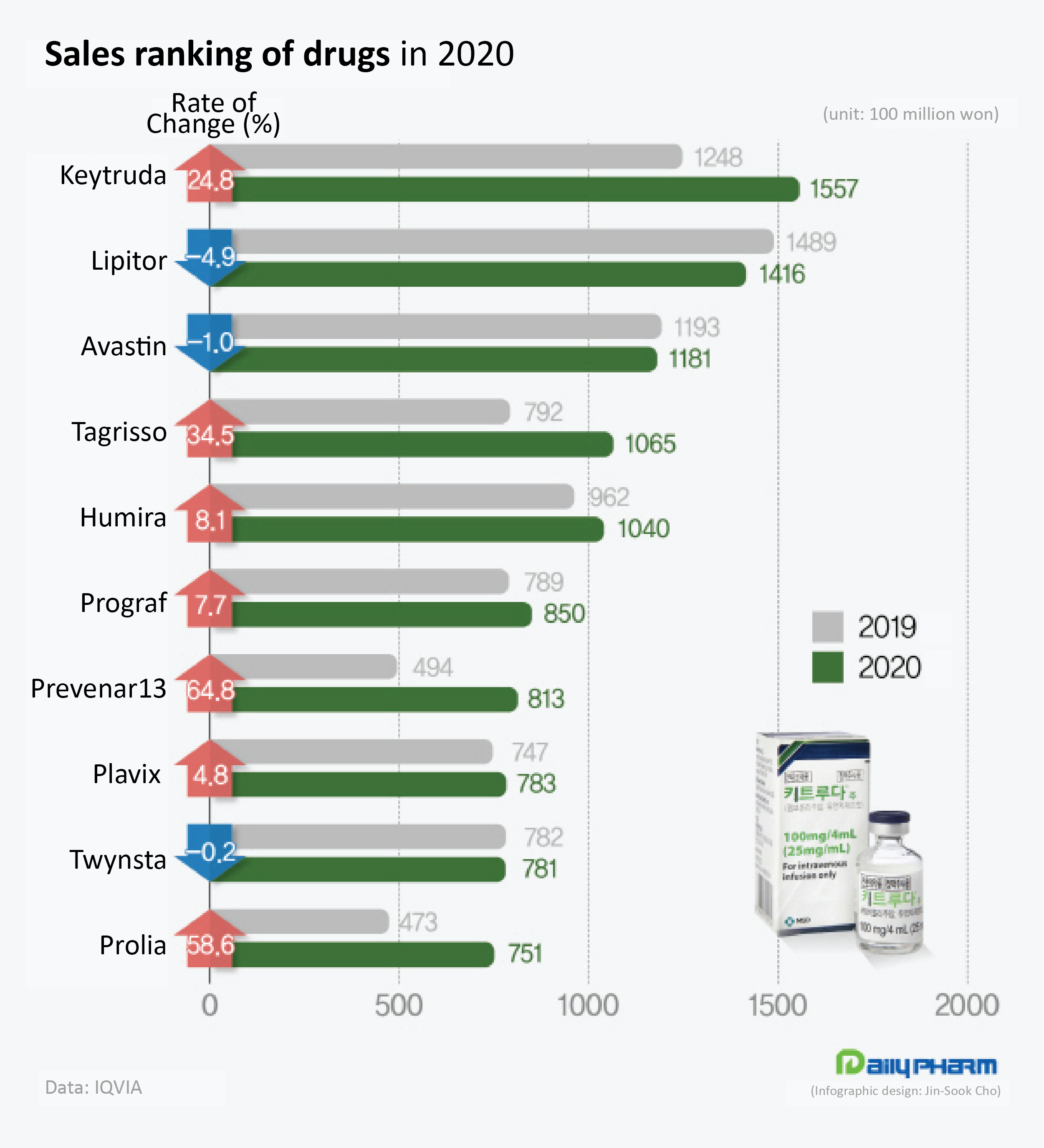
Keytruda recorded No.1 in global sales in 2020, earning $14.38 billion (₩16.8 trillion as of August 18th, 2021).
The same went for Korea as well.
According to IQVIA, Keytruda recorded ₩155.7 billion in the domestic pharmaceutical market in 2020 to rank first among all pharmaceuticals in sales and has maintained the lead in the first half of this year.
In the early years of its release, in 2016 and 2017, Keytruda sold around ₩10 billion’s worth; however, its sales hit ₩70 billion in 2018.
The upsurge was driven by its reimbursement approval as second-line treatment for NSCLC in August 2017.
As previously mentioned, much potential remains for Keytruda.
MSD is conducting more than 1,400 global clinical trials focusing on Keytruda.
Korea’s contribution is also quite significant in the area.
Around 120 of the global anticancer therapy trials being led by MSD are being conducted in Korea and consist of around 88% of all trials conducted by MSD Korea.
Based on the number of participating patients, Korea has ranked 1st among Asia-Pacific countries and 4th in global.
Reimbursement is an issue as the insurance benefit is only approved for two indications, as second-line treatment for melanoma and lung cancer.
The drug was not able to extend its scope of reimbursement since September 2017.
In MSD’s discussions with the health authorities to reimburse Keytruda as second-line treatment for lung cancer, its competitor, the ‘all-comer’ drug ‘Opdivo (nivolumab),’ took away a partial win by strategically narrowing its scope of reimbursement, however, Opdivo also had not seen much progress in expanding its reimbursement ever since.
The discussion for Keytruda’s reimbursement as first-line treatment in NSCLC has been ongoing for around 4 years now.
It took 9 attempts for the agenda to pass the Health Insurance Review and Assessment Service’s Cancer Disease Review Committee (CDRC) meeting, however, the committee still requested further revisions to be made to the cost-sharing plan.
With the burden of re-revisions in mind, MSD is waiting for the agenda to be put up for deliberation by the Drug Reimbursement Evaluation Committee.
With the negotiations for contract renewal of the Risk-sharing Agreement (RSA) also overlapping, Keytruda is now at its highest inflection point since its entry into the Korean market.
Due to its versatility, expanding the scope of reimbursement for Keytruda may indeed result in the creation of a budget-gobbling monster.
In every field, the industry always moves faster than the system.
Whether Keytruda will be able to continue making progress in the sea of unprecedented, advanced new drugs that are being introduced remains to be seen.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









