- LOGIN
- MemberShip
- 2025-12-25 20:56:11
- GS Cross’s Neulapeg sales jump fivefold in 3 years
- by An, Kyung-Jin | translator Alice Kang | 2021-08-31 06:14:16
The locally developed neutropenia treatment ‘Neulapeg’ is enjoying its belated prime.
The drug, which was unable to make a presence earlier at the time of its release is continuing to break new records every quarter since signing a sales partnership with Boryung Pharmaceuticals.
Neulapeg’s quarterly sales have risen over fivefold in the past 3 years, and are attempting to overtake the market leader position held by ‘Nelasta.’ According to the pharmaceutical market research institution IQVIA, sales of GC Cross’s ‘Neulapeg’ increased 64.8% YoY to reach ₩5.4 billion in Q2.
Compared to Q2 2018, quarterly sales of the product increased over fivefold.

The drug is used on cancer patients during chemotherapy to prevent side effects of decreasing immunity that occur from a decrease in the neutrophil level.
It received marketing authorization from the Ministry of Food and Drug Safety in August 2014 and began its sale in March of the next year.
‘Neulapeg’ applies the PEGylation technology that conjugates polyethyleneglycol to specific areas to improve the purity, stability, and half-life of the drug over existing therapies.
Neulapeg is considered a 2nd generation drug as may be administered only once per chemotherapy cycle to see an effect compared to existing neutropenia treatments that are administered 4-6 times per cycle.
Despite its strength of being nearly ₩300,000 cheaper than Kyowa Kirin’s ‘Neulasta(pegfilgrastim),’ Newlapeg wasn’t able to make much of a presence earlier at the time of its release.
In its third year, 2017, Neulapeg had sold ₩3.2 billion, and then ₩4 billion in 2018.
Quarterly sales of Neulapeg, which was less than ₩1 billion, started to soar after Boryung Pharmaceutical joined in as a sales and marketing partner with GC cross.
In Q4 2018, its sales jumped from ₩1.1 billion to 1.3 billion in the following quarter, 1Q 2019, and then continued upwards ever since.
GC Cross had signed a joint sales agreement for ‘Neulapeg’ with Boryung Pharmaceuticals in October 2018.
GC Cross had attempted to work with Boryung and utilize the company’s sales capability in the field of anticancer to expand its share in the neutropenia treatment market.
According to IQVIA, Neulapeg accumulated sales of ₩8.9 billion in 2019.
This is a 123.4% growth in sales in just one year after its partnership with Boryung Pharmaceuticals.
Last year, despite the spread of the COVID-19 pandemic, it increased its market share by twofold and raised ₩15 billion in sales.
This is a fourfold expansion in annual sales compared to 2018, before signing a partnership with Boryung Pharmaceuticals.
And the company is continuing to make new records, earning ₩4.9 billion in Q1, and ₩5.4 billion in Q2.
Cumulative sales in 1H of this year were ₩10.2 billion, a 62.8% increase from the previous year.
On the other hand, Neulasta sold ₩12.3 billion, a 0.2% decrease from the previous year.
This has narrowed the gap between the two products to ₩2.2 billion.
If this trend continues, experts believe the position of the two products may be reversed within this year.
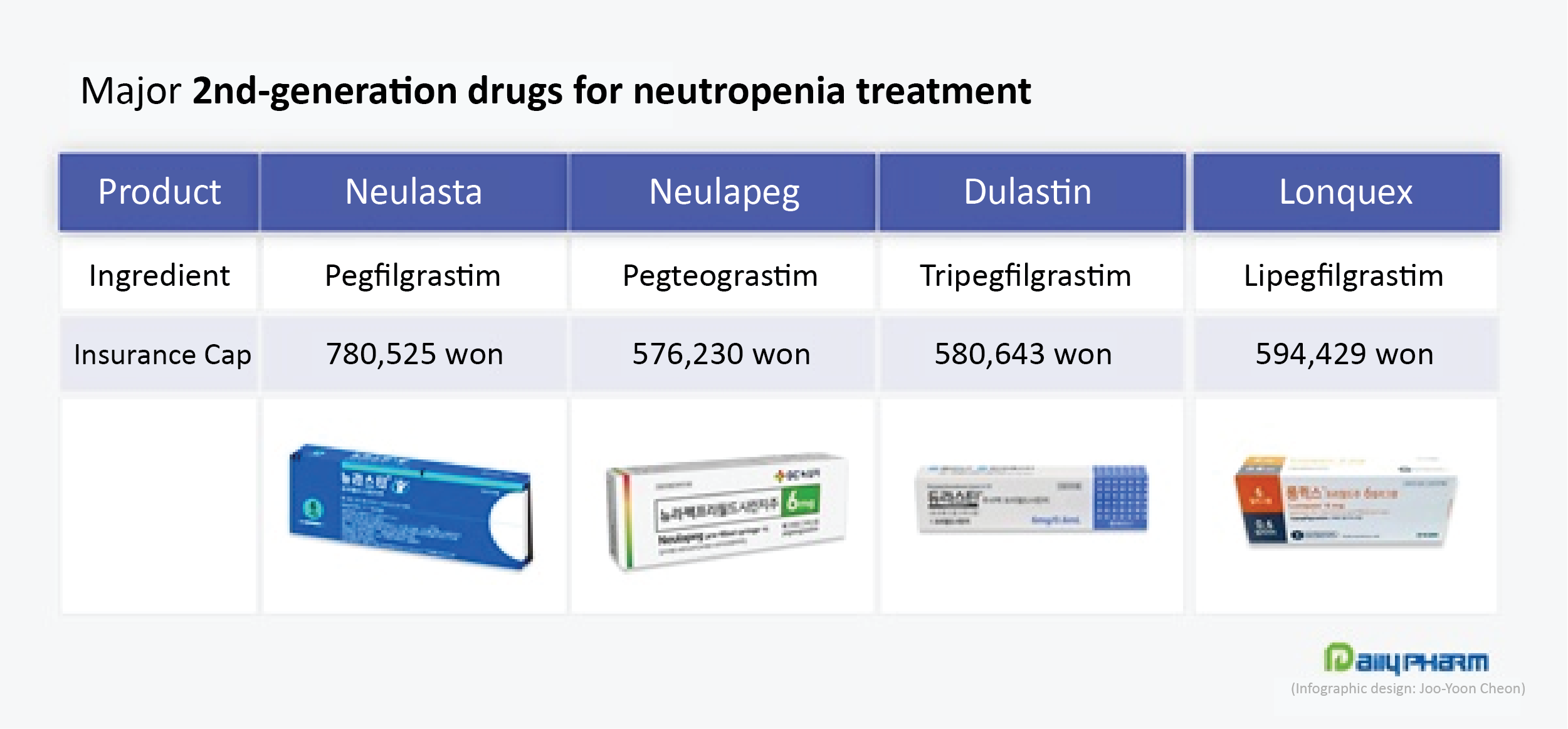
Dulastin sold ₩1.5 billion in 1H this year.
Until 2018, the drug had made sales similar to Neurapeg.
However, due to Neurapeg’s sudden and rapid growth in sales, the sales gap has widened to around 7 times.
Dong-a ST had received marketing authorization for Dulastin in August 2014, and has started selling it in March of the following year.
Dulastin is a second-generation G-CSF derivative that was applied to the company’s own extended-release technology for longer action.
Its extended-release technology allows for the drug to stay in the blood for a longer period of time, which allows patients to administer the drug only once per chemotherapy cycle.
‘Lonquex (lipegfilgrastim),’ another second-generation neutropenia treatment that was released in 2016, is also not seen much sales for many years.
Longquex sold ₩1.5 billion in 1H this year.
This was a 6.2% YOY increase.
Lonquex is a molecular structurally improved G-CSF that was developed by applying Handok Teva’s unique PEGylation technology.
It is also administered once per chemotherapy cycle.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









