- LOGIN
- MemberShip
- 2025-12-25 19:19:29
- Blockbuster anticancer drug series 4 - Avastin
- by | translator Choi HeeYoung | 2021-09-17 05:55:52
Avastin, a Vascal Endothermic Growth Factor (VEGF) inhibitor, which is also considered a good partner for immuno-cancer drugs.
It's the first anti-VEGF event to surpass 100 billion won.
Avastin was launched when Genentech in the United States was interested in angiogenesis research and discovered VEGF and genes.
Avastin has greatly succeeded along with new antibody drugs such as Rituxan and Herceptin.
After Avastin's approval, Roche completely acquired Genentec.
Avastin received a lot of expectations and concerns at once.
Avastin, which started as a treatment for colorectal cancer, had indications for various carcinomas such as breast cancer, lung cancer, and kidney cancer.
In particular, Avastin significantly increased the treatment effect in carcinoma.
However, side effects such as high blood pressure, blood clots, and heart failure caused by excessive inhibition of angiogenesis have become controversial.
Indications for breast cancer have been withdrawn in the United States due to ambiguous effects against toxicity.
Controversy also arose in unauthorized indications.
Wet macular degeneration, which causes blindness, is also caused by excessive proliferation of new blood vessels under the retina.
Avastin preference was much higher because of the low cost.
Avastin was widely used in the treatment of macular degeneration.
In Korea, Avastin has expanded its indications relatively smoothly to breast cancer, non-small cell lung cancer, kidney cancer, glioblastoma, ovarian cancer, and cervical cancer since it was approved as a treatment for colorectal cancer in 2005.
It was used as a primary treatment in all indications other than glioblastoma and became essential for chemotherapy.
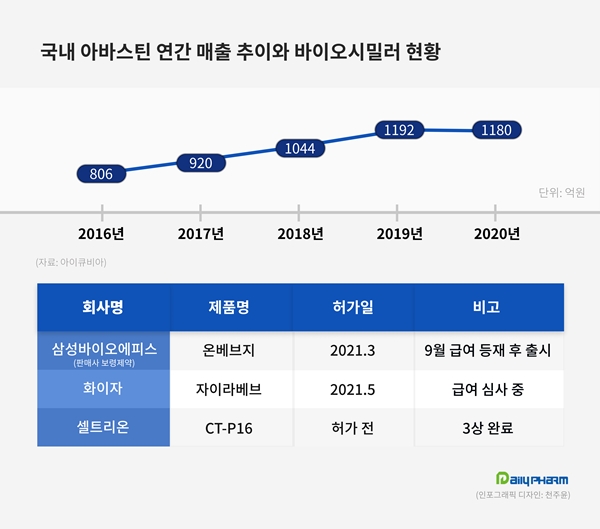
With the first registration in 2014, Avastin surpassed 100 billion won in sales for the first time in 2018, 13 years after approval based on IQVIA.
Last year, it posted 118 billion won in sales.
This is the third-highest selling figure in the entire drug market in Korea.
Will Avastin find a new opportunity? Avastin is a good drug to use with other anticancer drugs due to its mechanical nature.
It is used with conventional chemotherapy in all indications other than glioblastoma.
Targeted anticancer drugs have obtained primary treatment indications for EGFR-positive non-small cell lung cancer with good effects with Tarceva, an EGFR target treatment.
It is also used in primary maintenance therapy for ovarian cancer in combination with the PARP inhibitor Lynparza.
Roche received primary treatment indications for liver cancer and non-small cell lung cancer through combination therapy with its anti-PD-L1 immuno-cancer drug Tecentriq and Avastin.
Keytruda and Opdivo are also exploring the possibility of being used in combination with Avastin in various carcinomas.
It is expected that anti-VEGF drugs will compensate for the limitations of cancer immunotherapy alone.
Some clinical trials have failed, but combination of cancer immunotherapy and Avastin is still a good strategy.
Avastin is widely used.
Big Pharma such as Amgen, Pfizer, and Beringer Ingelheim are participating overseas, and two biosimilars have been released in Korea this year.
They are Onbevzi of Samsung Bioepis and Zirabev of Pfizer.
Onbevzi, the first simulator sold by Boryeong Pharmaceutical, has been paid since this month, and is in the midst of preparing for prescriptions at general hospitals.
However, Zirabev is more advantageous in indications.
Onbevzi, did not receive some indications for ovarian cancer related to the patent for use.
Celltrion and Prestige Biopharma are also developing biosimilar products, so competitive drugs are expected to increase further.
When competition begins in earnest, Avastin sales, which amount to 120 billion won, are expected to be inevitable.
Avastin sales have already declined as biosimilars have already entered the largest European and U.S.
markets.
Global sales fell 25% year-on-year last year.
In the case of Korea, drug prices have been continuously lowered since Avastin was registered, and the actual difference between the original and biosimilars is expected to be insignificant as only 5% of the patient's burden is applied.
The results may vary depending on the non-reimbursed item.
In particular, there are biosimilars that are cheaper than Avastin in off-label diseases such as macular degeneration.
Sales of expired patents are usually falling, but Avastin has new opportunities.
It is an expansion of benefits in combination with Tecentriq.
In the primary treatment of liver cancer, combined therapy passed the HIRA's Cancer Drugs Benefit Application Committee in February.
In particular, it is more difficult to replace biosimilars with combination therapy with immuno-cancer drugs.
The primary benefit for liver cancer is currently in the presumption stage for more than six months.
The key is how quickly Roche Korea and the government will be able to reach an agreement over Avastin drug prices, which are burdensome at high prices.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









