- LOGIN
- MemberShip
- 2025-12-25 18:58:06
- ‘Ibrance’ opens new horizon as the lead CDK4/6 inhibitor
- by | translator Alice Kang | 2021-10-14 05:38:35

The company had other excellent anticancer drugs such as ‘Xalkori,’ and ‘Sutene,’ but none had received as much spotlight as Ibrance.
As the ‘first CDK4/6 inhibitor,’ and ‘the first new breast cancer drug in 50 years,’ Ibrance had quickly rose to the ranks and became a blockbuster drug.
◆ The first CDK4/6 inhibitor, unrivaled status despite controversy Ibrance selectively inhibits Cyclin-dependent kinases (CDK) 4/6 that regulate cell division and cell growth to block the proliferation of tumor cells.
Lilly’s Verzenio (abemaciclib),’ and Novartis’s ‘Kisqali (ribociclib)’ belong to CDK4/6 inhibitor class drugs.
Among these drugs, Ibrance is the first drug in its class, the ‘first-in-class’ CDK 4/6 inhibitor.
Ibrance is indicated for the treatment of breast cancer in combination with an aromatase inhibitor as first-line endocrine therapy in postmenopausal women or combination with fulvestrant in pre-and post-menopausal women with disease progression following endocrine therapy.
For a year Ibrance was launched in Korea in August 2016, the field was full of expectations, disappointment, longing, and appeals.
The introduction of a new drug with a new mechanism of action was blissful news.
In particular, the patient population for Ibrance was wider than other drugs, as it targeted f hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, which accounts for 60% of all breast cancers.
These patients had to use anti-hormonal drugs such as aromatase inhibitors or chemotherapy, which had many systemic side effects if not managed until Ibrance appeared.
Before the introduction of Ibrance, this patient group had to use antihormonal therapy such as aromatase inhibitors, and if their condition was not managed with such therapies, use chemotherapy, which had many systemic side effects Korea was the fifth country to approve the drug.
In December of the same year, the drug's efficacy and safety in Asian patients were verified through a clinical trial and rose as the drug that could change the breast cancer treatment paradigm.
The National Comprehensive Cancer Network’s ‘Category 1’ recommendation of CDK4/6 inhibitors as combination therapy for the treatment of HR+/HER2- advanced/metastatic breast cancer had also contributed greatly to establishing the drug as standard care in breast cancer.
However, Ibrance did not immediately rise and become the star after its release.
The drug had suffered receiving reimbursement due to its high drug price, costing over ₩5 million per month.
In particular, 2017 was the time when many high-price anticancer drugs were introduced and rejected reimbursement, and Pfizer Korea was put at a difficult price due to its difficulty in demonstrating cost-effectiveness, and the issue that Ibrance was more expensive in Korea than abroad.
The Health Insurance Review and Assessment Service recognized Ibrance’s feasibility of reimbursement in July 2017, after rounds of review.
However, the approval left much to be desired among patients because the reimbursement was only allowed as first-line endocrine therapy in postmenopausal women in combination with letrozole.
Its use as second-line therapy in combination with fulvestrant was non-reimbursed, and premenopausal patients, which are much more prevalent in the East than in the West, were unable to receive the reimbursement benefit at all.
The 2nd-line reimbursement of Ibrance+fulvestrant was only approved last July, 4 years after Ibrance’s approval.
In addition, the fact that the drug was reimbursed at the same time with the CDK4/6 class latecomer Verzenio, left much to be desired for Ibrance.
However still, Ibrance is fully exerting its strength as the ‘first’ introduced to its market.
Among the three products in the CDK4/6 market, Ibrance has been dominating the market as the second drug, Verzenio, was approved in Korea three years later than Ibrance, in May 2019.
The third CDK4/6 inhibitor Kisqali was approved 5 months after Verzenio, in October 2019 and listed for reimbursement in November last year.
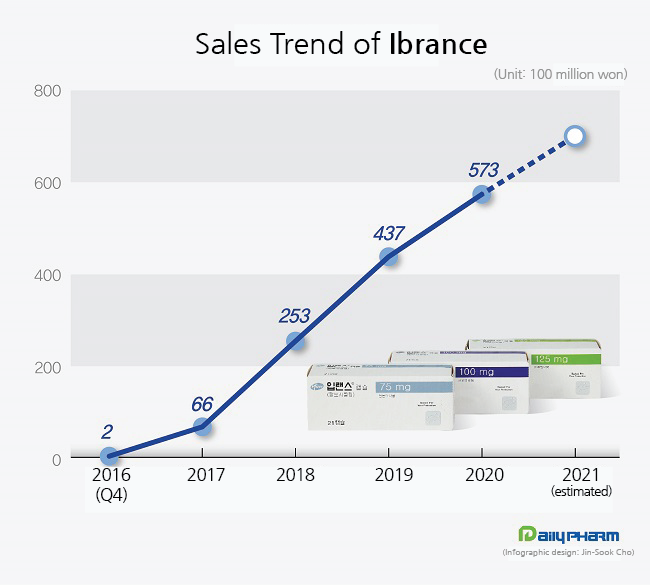
According to the market research institution IQVIA, sales of Ibrance, which was ₩6.6 billion in 2017, surged 283% in 2018 to reach ₩25.3 billion after being listed for reimbursement.
Then, sales rose 73% again to record ₩43.7 billion in 2019.
Last year, Ibrance sold around ₩60 billion and is expected to sell near ₩70 billion this year.
Compared to Ibrance’s quarterly sales, which is around ₩15 billion, Verzenio’s sold ₩3 billion, and Kisqali sold mid-₩1 billion range.
◆Three-way competition starts now… data will decide the winner With all three products now approved for reimbursement, the competition is finally in full pace.
Of course, the other two drugs have a long way to go because they need to break down the solid position held by Ibrance in the market.
Therefore, the latecomer drugs are betting in areas where Ibrance has not reached, as preoccupying new areas is easier than breaking down Ibrance's established position.
The attempt will also support the expansion of the overall CDK4/6 inhibitor market amid the increased introduction of new-class drugs.
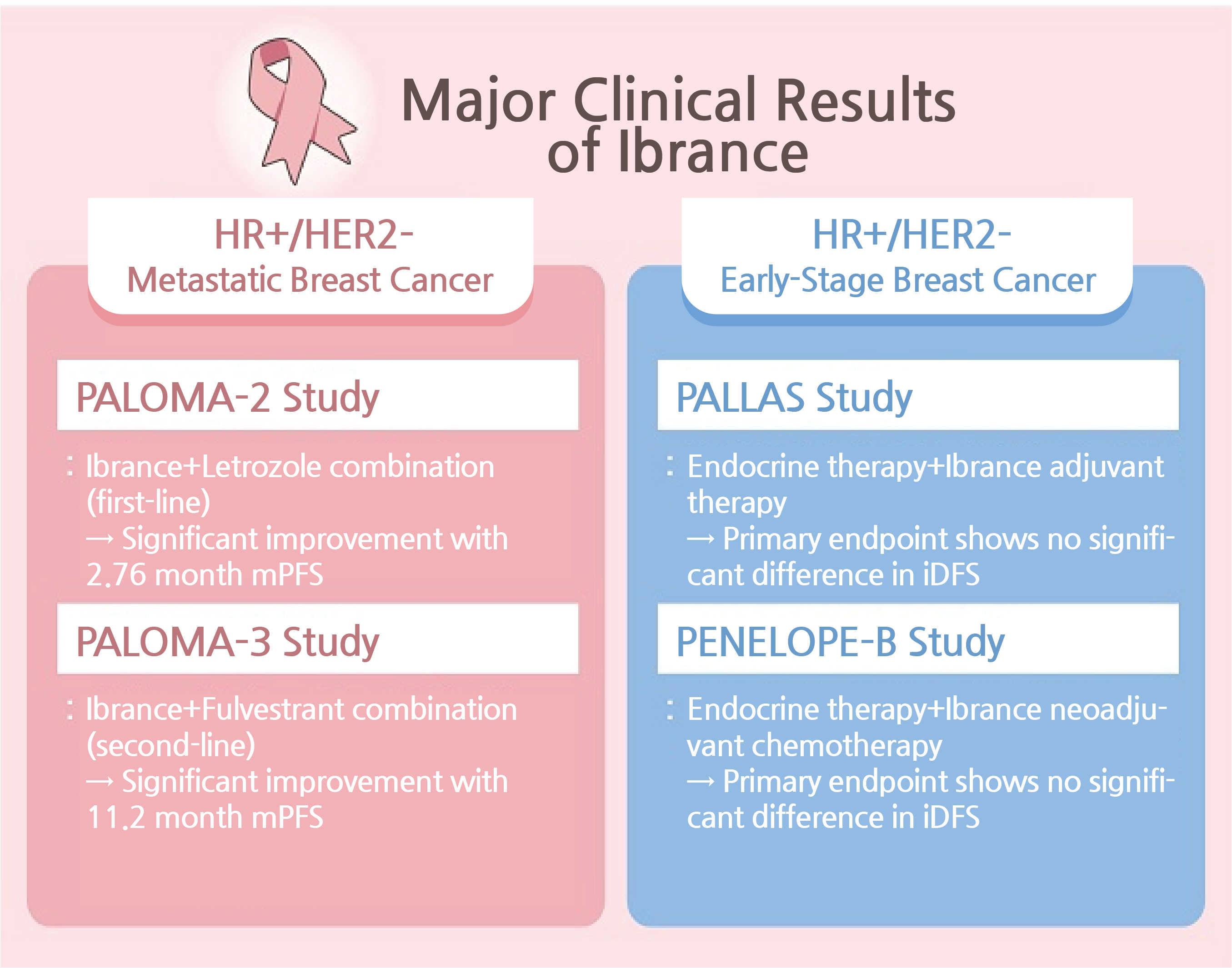
Despite its strength in metastatic breast cancer, Ibrance did not show as a good result in early breast cancer.
In both the PALLAS study that tested Ibrance+endoctrine therapy as adjuvant therapy in hormone receptor-positive (HR+)/HER-2 negative (HER2-) metastatic breast cancer and the PENELOPE-B study that tested Ibrance+endoctrine therapy after neoadjuvant chemotherapy, Ibrance did not show a difference in invasive disease-free survival (iDFS) compared to placebo (primary endpoint).
Pfizer believed the high early discontinuation rate due to the strict dose restriction and the short period of administration had an effect.
On the other hand, Verzenio achieved its primary endpoint in a median follow-up period of 15.5 months as adjuvant therapy, suggesting the potential for scope expansion.
Of course, whether using CDK4/6 inhibitor as adjuvant therapy in early breast cancer patients is more beneficial, remains a question that needs to be addressed.
Kisqali can be used in combination with an aromatase inhibitor as first-line endocrine therapy in pre/perimenopausal women that cannot use Ibrance, and therefore may resolve the unmet needs of Ibrance.
Also, Kisqali has demonstrated the longest overall survival period among all drugs, threatening Ibrance’s sole lead in the market.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









