- LOGIN
- MemberShip
- 2025-12-25 15:33:02
- The kit for precocious puberty in Korea has been stopped
- by Moon, sung-ho | translator Choi HeeYoung | 2021-12-08 06:00:17

As the number of patients, which was 80,000 in 2015, exceeded 130,000 in 2020, there are opinions that it should now be recognized as a social problem.
In response, some in the medical community and clinical sites predict that the number of precocious puberty patients will increase further as outdoor activities decrease due to the prolonged COVID-19 pandemic.
Amid this situation, it has been confirmed that confusion is occurring at front-line medical sites due to the recent suspension of the supply of "diagnostic reagents" that can identify patients with precocious puberty.
With the suspension of the supply of diagnostic reagents, which were the only health insurance coverage targets, even the diagnosis of precocious puberty patients, which is rapidly increasing due to prolonged COVID-19, is developing.
According to the medical and pharmaceutical industries on the 4th, clinical doctors are suffering from the suspension of supply of injection items that are prescribed precocious puberty as a "diagnostic reagent" since March.
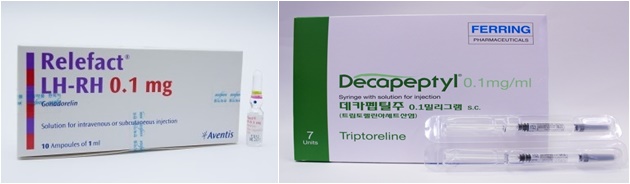
The item is Relefact LH-RH supplied by Handok in Korea.
As Sanofi, the original developer, decided to suspend the supply of this item in March, stock of reagents for diagnosing precocious puberty has disappeared in Korea.
According to the MFDS, the import performance of Relefact LH-RH in 2020 is about 1.132.26 billion won.
Considering that it was 650.58 million won in 2015, the performance has increased, but it is lower than expected.
The problem is that Relefact LH-RH is the only item as a diagnostic reagent for precocious puberty.
Lutrelef of Perring Pharmaceutical Korea, which can be said to be the same ingredient, has already been withdrawn from the domestic market due to the expiration of its validity period.
For this reason, medical sites are unable to diagnose precocious puberty patients due to a lack of diagnostic reagents.
In the diagnosis of precocious puberty, LH and FSH are measured in the blood for 2 hours at intervals of 15 to 30 minutes after administration of "sexual stimulation hormone secretion hormone." If the maximum concentration of sulfonation hormone is 5 IU/L or higher, it is judged to be activated and diagnosed with precocious puberty.
As Relefact has not been imported into Korea, it is difficult to diagnose precocious puberty nationwide, said a professor of pediatrics in a province who asked for anonymity.
"As the number of precocious puberty patients has already increased rapidly through the COVID-19 pandemic, I wonder what preparation the health authorities have made." It is prescribed as an "off label" for precocious puberty, which has become difficult to diagnose.
If so, how is the medical field responding to the suspension of the supply of diagnostic reagents?
As a result of the coverage, it was found that some hospitals and clinics are prescribing other drugs offline as the supply of Relefact has been stopped.
In fact, it has been confirmed that some hospitals and clinics apply for approval for excessive use of Ferring's Decapeptyl Depot and use it as a reagent for diagnosing precocious puberty.
Decapeptyl is a specialized drug licensed for hormone-dependent prostate cancer, endometriosis and uterine myoma, central puberty early onset in girls under the age of 9 and boys under the age of 10.
Another professor who runs a clinic said, "As papers have been presented that they have obtained good results in Thailand, some hospitals in Korea are already receiving health insurance benefits by submitting approval to the HIRA for the use of non-reimbursed drugs beyond the scope of reporting." In fact, the HIRA official also said, "As the supply of Relefact injections has been suspended, off-label use through Decapeptyl is being carried out.
Currently, we are considering approving the use of hospitals and clinics that submit overseas papers." It was found that the seriousness of the problem was recognized and countermeasures began to be prepared, focusing on related societies such as the Korean Society of Pediatric Endocrinology.
If it is no longer possible to secure Relefact, the use of off-label for Decapeptyl is suggested as an alternative, and some pharmaceutical companies are requesting the re-import of related items.
It is only hoped that the suspension of supply will end in a short period of time as a pharmaceutical company that resupplies the drug to Korea.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









