- LOGIN
- MemberShip
- 2025-12-25 10:14:10
- Global lead Humira’s sales drop... biosimilars occupy 16%
- by Chon, Seung-Hyun | translator Kang, Shin-Kook | 2022-03-11 06:04:50
The ₩200 billion autoimmune disease market has finally revealed its form in Korea.
The growth of the global leading product ‘Humira’ had fallen somewhat due to the introduction of its biosimilars.
The 5 biosimilars by domestic companies Celltrion, Samsung Bioepis, LG Chem, etc.
are also increasing their influence in the market, but their total market share remains in the 10% range.
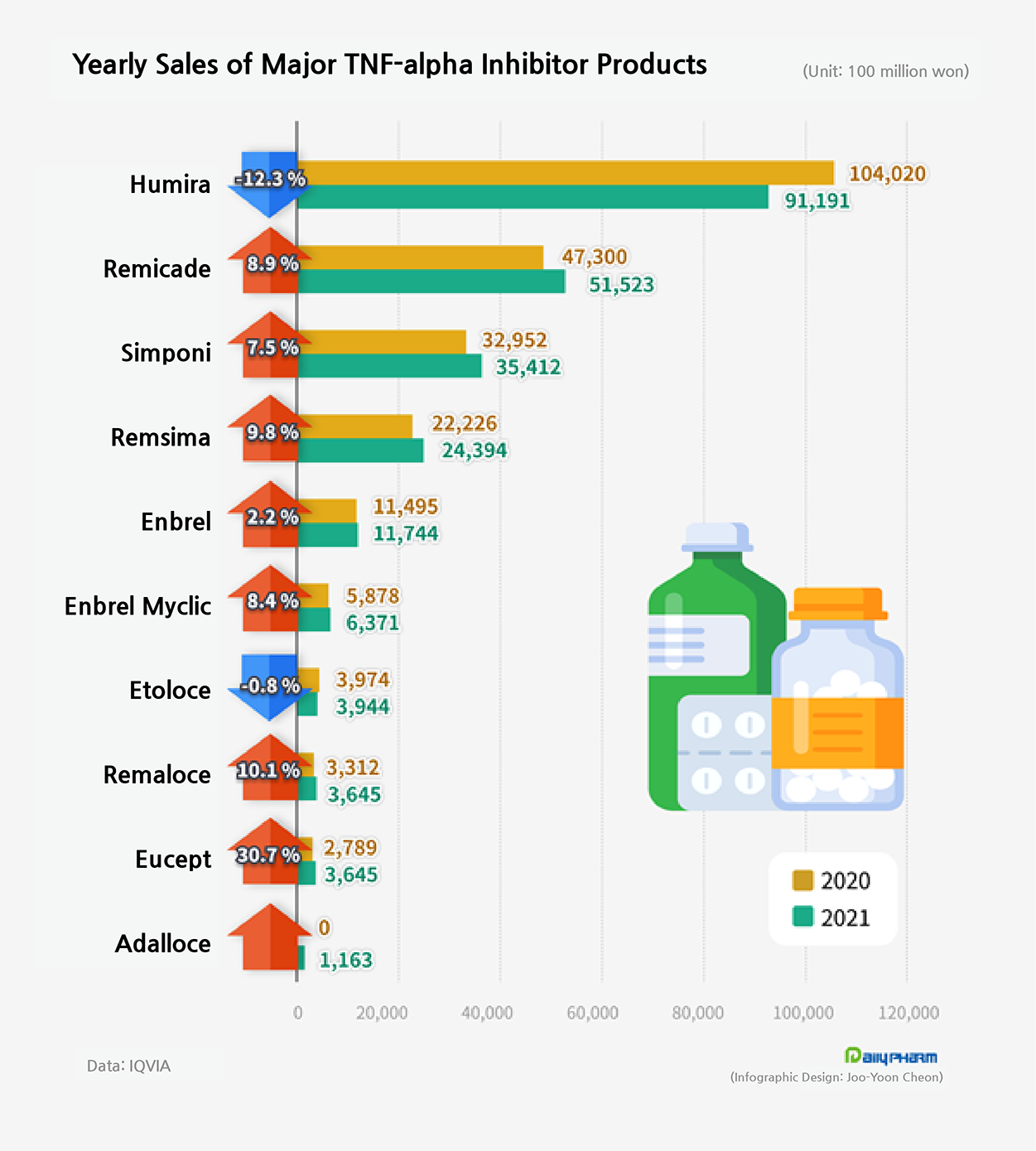
◆ TNF-α inhibitor market shrinks for the first time… aftermath of Humira’s drug price cut According to the market research institution IQVIA on the 9th, sales in the domestic TNF-α inhibitor market was ₩233 billion, a 0.4% decrease from the previous year.
TNF-α inhibitors are antibody drugs that suppress the expression of tumor necrosis factor TNF alpha in the body and are prescribed for autoimmune diseases such as rheumatoid arthritis, Crohn's disease, and ulcerative colitis.
Products from multinational pharmaceutical companies such as Humira, Remicade, Simponi, Enbrel, and Enbrel Myclic are leading the market, and biosimilars from domestic companies such as Celltrion, Samsung Bioepis, and LG Chem have also thrown their hats into the market.

The market had continued to show high growth every year since making a twofold growth in 6 years from ₩120.2 billion in 2014 to ₩233.9 billion in 2020.
However, the market showed sluggish growth after 2018, when it recorded a 23.2% YoY growth, then grew 11.2% and 4.5% respectively in 2019 and 2020.
The market turned downwards for the first time last year.
The recent sluggish market growth of the TNF-α inhibitor market is due to the sluggish sales of the lead product in the market - Humira.
Humira sold ₩91.2 billion last year, a 12.3% decrease from the previous year.
This is the first time Humira’s sales fell compared to the previous year.
Since its release in 2006, its sales rose every year to exceed ₩100 billion for the first time in 2020 but then fell to a record ₩90 billion the next year.
The sales decline is due to the introduction of biosimilars.
Samsung Bioepis had registered its Humira biosimilar ‘Adaloche’ in May last year and released it in the domestic market.
In principle, the insurance price cap of original drugs falls 30% with the introduction of biosimilars under the Korean drug pricing system.
Humira’s price had been reduced by 30% from June 7th last year.
With the price cut, prices of three drugs - Humira Pen inj.
40mg/0.4mL, Humira Prefilled syringe inj.
40mg/0.4mL, Humira inj.
40mg vial fell 30% from ₩411,558 to become ₩288,091, and Humira Prefilled syringe inj.
20mg/0.2mL fell from ₩224,002 to ₩156,801.
Humira recorded ₩27.5 billion in sales in Q1 last year but dropped 24.9% to record ₩20.7 billion in just the one quarter since.
Humira then made some recovery to ₩21.1 billion and ₩21.9 billion in Q3 and Q4 but wasn’t able to recover from the impact of their price cuts.
Sales of other TNF-α inhibitor products of multinational pharmaceutical companies such as Janssen’s Remicade and Simponi, Pfizer’s Enbrel, and Enbrel Myclic have shown a continuous increase.
◆ Domestically developed similars make ₩36.8 billion...
twofold increase in 4 years but occupy only around 10% of the market Although domestic biosimilar products are gradually expanding their market share in the TNF-α inhibitor market, their market influence is still not large.
Remsima and Remaloce are Remicade biosimilars.
Originals of Adaloche and Eucept are Enbrel.
Adaloche is Humira’s biosimilar.
Last year, sales of the 5 TNF-α inhibitor biosimilars increased 13.9% from the previous year to record ₩36.8 billion.
This is over a twofold increase in 4 years from the ₩18.2 billion in 2017.
Remsima, which first appeared in 2013, is leading the growth of biosimilars.
Remsima's sales last year were ₩24.4 billion, up 9.8% from the previous year.
Remsima’s sales decreased 12.0% from ₩25.3 billion in 2019 to ₩22.2 billion the following year, then made a rebound last year.
Biosimilars of Samsung Bioepis and LG Chem barely make less than ₩50 billion in annual sales.
Samsung Bioepis’ Etoloce recorded ₩3.9 billion in sales last year, down 0.8% from the previous year.
It recorded a high growth rate from the ₩700 million in 2017 to ₩2 billion in 2018, then to ₩4 billion in 2020, but then sales stalled the last year.
Remaloce’s sales increased 10.1% from the previous year to record ₩3.6 billion but did not significantly influence the total market.
LG Chem’s Eucept had also shown 30.7% growth in the market recording ₩3.6 billion in sales but did not exert much influence in the market.
The 5 domestically developed biosimilars in the TNF-α inhibitor market only was able to occupy 15.8% last year.
In 2020, its sales grew 13.8%, a slight increase, which is in stark contrast to its big success made in Europe and the US.
In Korea, the difference in the insured drug prices between original drugs and biosimilars is not large, therefore the latecomers cannot penetrate the market as quick as in Europe or the United States.
Some analysts say that it is not easy overcome the trust in original drugs with biosimilars that have been established for a long time as the drugs are used in critically ill patients.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









