- LOGIN
- MemberShip
- 2025-12-20 08:39:52
- Various immunotherapies attempt to treat Alzheimer’s
- by Son, Hyung Min | translator Alice Kang | 2025-07-15 06:06:17
A series of immunotherapy candidates are showing promise in the field of Alzheimer's disease treatment.
With the limitations of existing antibody therapies becoming apparent, multinational pharmaceutical companies are attempting approaches targeting new immune pathways.
In particular, pipelines targeting innate immune regulation, such as NK cell therapies and TREM2 antibodies, are gaining attention.

This is a case approved under the U.S.
Food and Drug Administration's (FDA) Compassionate Use program, applied to patients whose cognitive decline persisted despite treatment with the amyloid-targeted therapy lecanemab.
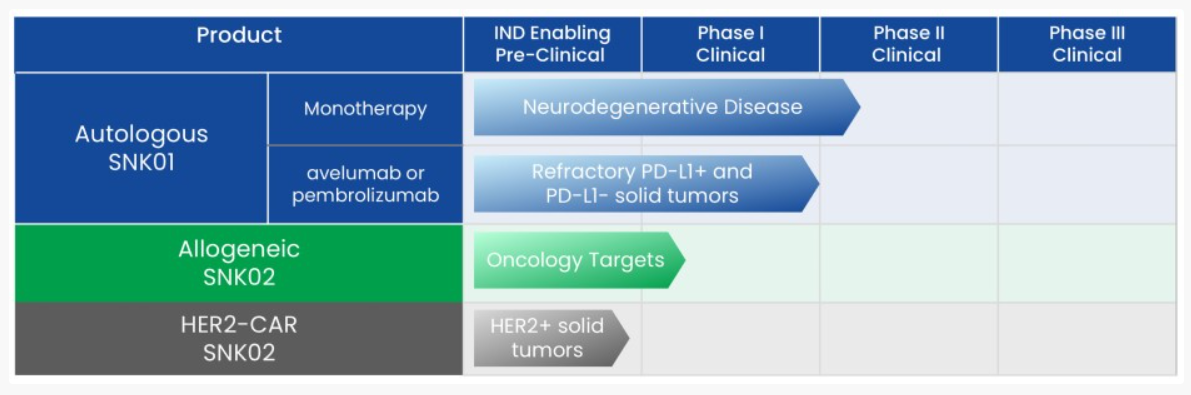
Troculeucel, NKGen’s cell therapy developed by using non-genetically modified ex vivo expanded autologous NK cells, is currently in a Phase IIa clinical trial in the U.S.
targeting moderate patients.
NKGen stated, “The therapy demonstrated efficacy in improving amyloid, tau, and alpha-synuclein levels in cerebrospinal fluid (CSF) by crossing the blood-brain barrier (BBB), as well as reducing neuroinflammatory markers such as GFAP.” In the previously conducted Phase I clinical trial, troculeucel demonstrated cognitive function stabilization and improvement in approximately 70% of patients, along with changes in biomarkers such as reduced pTau181 and GFAP levels in CSF.
Treatment responses were dose-dependent, and no significant adverse reactions were observed.
While existing antibody therapies have only slowed the progression of cognitive decline, NK cell therapy targets both inflammation control and protein aggregation resolution, opening up the possibility of a new mechanism of action for use.
In particular, its use in patients unresponsive to existing drugs suggests potential as part of a combination strategy or as a new treatment option.
Novartis recently began a multinational Phase II clinical trial of VHB937, a TREM2 antibody-based drug candidate, in multiple countries, including South Korea.
This study is designed as a randomized, placebo-controlled trial to evaluate the efficacy and safety of VHB937 in patients with early-stage Alzheimer's disease over 72 weeks.
VHB937 increases the expression of TREM2, a surface receptor on dendritic cells, and inhibits its shedding.
According to the company, this strategy aims to enhance intracellular signaling, thereby simultaneously targeting the phagocytic function of microglia and anti-inflammatory effects.
Sanofi recently acquired Vigil Neuroscience, a company developing a TREM2 small molecule agonist, and entered the field in earnest.
VG-3927, which Vigil is developing, binds only to cell membrane receptors and does not bind to soluble TREM2, enabling more precise regulation of microglia function, which is a key differentiator.
Sanofi plans to begin Phase II clinical trials of VG-3927 in the third quarter of this year.
Targeting TREM2 is a mechanism of action that leading companies such as AbbVie and Takeda have experienced a series of failures.
However, latecomers are shifting their focus to stabilization strategies and small molecule mechanisms after carefully analyzing the reasons for previous failures.
New mechanisms continue to be developed in Korea as well
SNR1611 (generic name: trametinib), developed by the Korean drug development company Genuv, is one representative example.
SNR1611 is a repurposed small-molecule compound derived from the already-approved anticancer drug trametinib.
It works by inhibiting MEK1/2 enzymes within the MAPK/ERK signaling pathway.
Through this mechanism, it suppresses the aberrant activation of MAPK signaling observed in neurodegenerative diseases such as Alzheimer’s disease, thereby promoting neural stem cell differentiation and the generation of new neurons.
Recent preclinical studies have shown that SNR1611 promotes the expression of specific genes involved in brain neuron differentiation, resulting in a more than 2.7-fold increase in brain neural regeneration and approximately a twofold improvement in memory.
These findings were published in “Experimental & Molecular Medicine,” a sister journal of the prestigious scientific journal Nature, thereby establishing scientific evidence.
When SNR1611 was administered to an animal model with Alzheimer's disease, neural regeneration increased by approximately 176% and 295% in the dentate gyrus and subventricular zone, respectively, which are critical regions for memory formation.
Additionally, neural regeneration effects were confirmed in the cerebral cortex, and memory improved by nearly twofold in the treatment group.
Notably, SNR1611 has drawn attention for its ability to induce neurogenesis—a process previously considered nearly impossible in the adult brain—through pharmacological intervention.
Genuv is also developing SNR1611 as a treatment for amyotrophic lateral sclerosis (ALS).
In ALS animal models, administering SNR1611 reduced abnormal protein aggregation through autophagy activation and confirmed motor neuron protection effects.
Currently, Phase 1/2a clinical trials for ALS are underway at five major hospitals in South Korea.
AriBio is developing AR1001, a new drug candidate for Alzheimer's disease.
In March, the company successfully transferred the technology to China's Q-BioTech.
AR1001 targets multiple causes of Alzheimer's disease, including PDE5 and toxic proteins.
This new drug candidate is an improved oral treatment based on mirodenafil (Mvix), a medication similar to Viagra for erectile dysfunction.
Recent findings suggesting that PDE5 inhibitors like Viagra and Cialis may prevent Alzheimer's disease provide further evidence supporting the mechanism of AR1001.
AR1001 is currently in Phase III clinical trials.
AriBio has initiated global Phase III clinical trials in the United States, where the first patient dosing began in December 2022, as well as in Korea, the United Kingdom, Germany, France, Spain, Italy, Denmark, the Netherlands, the Czech Republic, and China.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










