- LOGIN
- MemberShip
- 2025-12-25 06:25:35
- Avastin’s Q1 sales drop sharply… future sales prospects?
- by | translator Alice Kang | 2022-05-25 05:47:51
The drug had been much influenced by the drug price cut that followed the introduction of its biosimilars.
However, whether the drop will continue remains to be seen due to the rise of new variables from the additional price cut applied recently, the indication expansion to liver cancer, and the possibility of intensified competition among biosimilars this year.
◆Avastin’s sales drop sharply for 2 consecutive quarters… due to a 30% price cut According to the pharmaceutical market research institution IQVIA, Avastin’s sales in Q1 this year were ₩19.3 billion, a 32.6% YoY decrease from the ₩28.7 billion it made in the same period last year.
Avastin is a blockbuster drug that has made ₩100 billion in annual sales over the past 5 years.
Avastin inhibits VEGF, the main protein involved in inducing angiogenesis to create an antitumor effect.
It is used in various cancer types including breast cancer, non-small cell lung cancer, renal cell carcinoma, and ovarian cancer.
Avastin’s sales, which recorded ₩92 billion increased to ₩104.5 billion in 2018, and then ₩119.3 billion the next year.
However, sales fell to ₩118.1 billion in 2020 and then dropped 4.9% last year to record ₩112.3 billion.
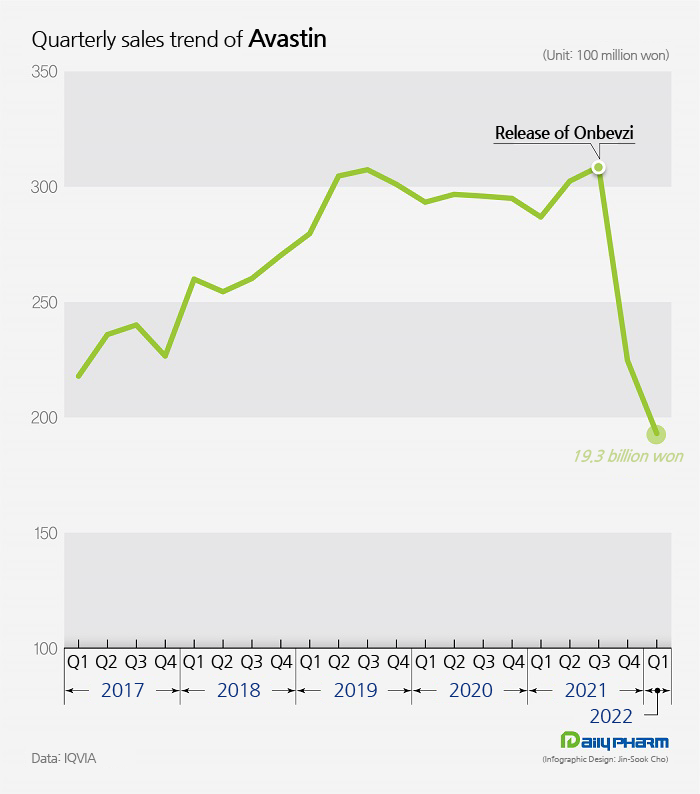
Avastin's sales, which reached ₩30.9 billion in Q3 last year, fell 27.2% to ₩22.5 billion in Q1 this year.
Then, in the first quarter of this year, it fell to ₩19.3 billion.
This is the first time Avastin’s quarterly sales recorded less than ₩20 billion in the past five years.
The sales drop was greatly influenced by the price cut that was made due to the introduction of its biosimilars.
Samsung Bioepis received approval for the first Avastin biosimilar ‘Onbevzi’ in March last year and entered the market in earnest after being listed for insurance reimbursement in September.
With Onbevzi’s listing, Avastin’s price ceiling fell 30% in October last year.
Price of Avastin 0.1g/4mL fell from ₩330,387 to ₩231,271 and Avastin 0.1g/16mL fell from ₩1,077,531 to ₩752,746, respectively.
In other words, the company received a sales hit corresponding to the 30% price cut.
Avastin’s sales drop increased greater with Onbevzi generating ₩1.8 billion in sales in Q1 this year, and Samsung Bioepis is attempting to expand its market and signed a co-promotion agreement for the sale of Onbevzi in Korea.
Onbevzi’s price was set at 63% of Avastin’s price, at ₩208,144 (0.1g/4mL) and ₩677,471 (0.4g/16mL).
◆Reimbursement expansion, additional price cuts, intensified competition….entangles the bevacizumab market Although Avastin’s sales dipped in the recent 2 quarters, whether the drop will continue remains unknown.
For one, Roche received reimbursement approval for the combination of Avastin and its cancer immunotherapy ‘Tecentriq’ in liver cancer.
In addition, other variables such as the additional price cuts, difference in price with its biosimilars, entry of additional competitors, etc had risen recently.
On April 29th, the Ministry of Health and Welfare assigned reimbursement standards for the Tecentiq+Avastin combination therapy in the first-line treatment of hepatocellular cancer.
This is the first time reimbursement was applied to a combination therapy that uses cancer immunotherapy in liver cancer.
The Tecentiq+Avastin combination therapy may now be used as a first-line treatment option with other existing first-line treatments – Nexavar and Lenvima.
This reimbursement extension has laid the foundation for Avastin to rebound in sales.
Also, the reimbursement extension had made Avastin’s drug price similar to its biosimilars.
With insurance benefits extended to liver cancer, the price ceiling of Avastin has been reduced by an additional 5% since October last year.
From this month, the price ceiling of Avastin will be ₩218,782 (0.1g/4mL) and ₩712,098 (0.4g/16mL).
The price difference between the 4mL doses is only ₩1,638 and ₩34,627 for the 16 mL dose.
This reduced gap in price is analyzed to have decreased the biggest strength held by its biosimilars – the price competitiveness.
On the other hand, Avastin’s biosimilar market has been unfolding in a more complex manner.
The industry prospects are that Pfizer's Zirabev which was approved in May last year, and Alvogen’s Alymsis which was approved in January will join in the competition this year.
On top of that, Celltrion also applied for permission for its 'CT-P16' in October last year.
The Avastin biosimilar market, which had only one product, has quickly formed a multilateral competition structure.
In particular, each of the Avastin biosimilars is showing a difference in price and its indications, and some biosimilar developers are in a patent dispute with the original company over some indications, including ovarian cancer.
Recently, Alvogen lost to Roche in one patent invalidation trial.
Its impact on the original Avastin will vary depending on the outcome of further lawsuits.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









