- LOGIN
- MemberShip
- 2025-12-25 02:53:05
- Exports of CKD·Dong-A ST's antianemic biosimilars increase
- by Chon, Seung-Hyun | translator Alice Kang | 2022-06-10 05:43:27
The biosimilar products developed by Chong Kun Dang and Dong-A ST are starting to make their way into the overseas market, with its Nesp biosimilars starting to generate sales in earnest abroad.
Although the companies’ products did not show explosive growth upon entry, the products are increasing their presence in the market.
According to the Financial Supervisory Service, Chong Kun Dang’s export sales recorded ₩19.4 billion in Q1 this year, a 57.7% increase compared to sales made in the same period of the previous year.
Compared to the ₩10.6 billion it had made in Q1 2020, the scale of exports increase 82.3% in two years.
In the case of Chong Kun Dang, the company’s sales had been solely dependent on domestic demand.
Its exports in Q1 2019 were a mere ₩6.3 billion.
Chong Kun Dang’s ‘Nesbell’ is being highlighted as the driver of CKD’s recent growth in exports.
New drug products being developed by Hanmi Pharmaceutical have received a total of 20 orphan drug designations from regulatory authorities in Korea and abroad.

Idiopathic pulmonary fibrosis is a rare condition caused by an unknown pulmonary inflammatory process and fibroblast hyperproliferation.
Patients with IPF experience a rapid decline in lung function from tissue fibrosis and even death.
Although it occurs in less than 100 cases per 10,000, its treatment had been rendered difficult due to the lack of efficacy in its approved treatments.
LAPS Triple Agonist is a triple-action biologic drug that activates GLP-1, Glucagon, and GIP.
It simultaneously targets▲ Glucagon, which inhibits fibrosis ▲ GLP-1, which facilitates insulin secretion and helps suppress appetite, and ▲ GIP, which facilitates insulin secretion and anti-inflammatory effect.
Hanmi Pharmaceutical had confirmed its drug’s antiinflammatory and antifibrotic in animal models with idiopathic pulmonary fibrosis.
With the designation Hanmi Pharmaceutical received a total of 20 orphan drug designations for its 10 indications in 6 pipelines (9 FDA designations, 8 EMA designations, and 3 Korea MFDS designations).
LAPS Triple Agonist received a total of 6 orphan drug designations from both the FDA and EMA for the treatment of ▲primary biliary cholangitis, ▲primary sclerosing cholangitis, and ▲IPF.
The FDA and EMA grant orphan drug designations to facilitate smooth development and approval of drugs that treat rare, incurable, or life-threatening diseases.
In Europe, drugs that receive the designation pay reduced fees for marketing-authorization applications and may benefit from ten years of market exclusivity once they receive marketing authorization in the European Union (EU) An official from Hanmi Pharmaceutical said, “All the indications that the drug received orphan drug designation on induces fibrosis in specific tissues and has high unmet needs.
LAPS Triple Agonist’s designation has meaning in that the drug's innovativeness is receiving attention from regulatory agencies in advanced countries.
Nesbell is a biosimilar of the second generation anemia treatment ‘Nesp (darbepoetin-α).’ It is prescribed to treat ▲anemia in chronic kidney disease patients and ▲anemia in patients with solid cancer who receive chemotherapy.
Chong Kun Dang received marketing approval from Japan's Ministry of Health, Labor, and Welfare in September 2019.
The Japanese subsidiary of U.S.
global pharmaceutical company Mylan N.V.
is in charge of sales in Japan.
Analysts believe that the continued growth of Nesbell’s sales in Japan had driven the continued growth of Chong Kun Dang’s export in Japan.
Nesbell holds great significance for the company as it is the first biosimilar that it succeeded in developing.
Chong Kun Dang entered the biosimilar market after securing its own platform technology in 2008.
After initiating a Phase I trial on Nesbell in 2012, Chong Kun Dang succeeded in becoming the first company to commercialize a biosimilar of Nesp by receiving marketing approval for Nesbell from the Ministry of Food and Drug Safety at the end of 2018.
Nesbell is also set to enter the Middle East soon.
Chong Kun Dang signed an export agreement for Nesbell with an Oman company Menagene Pharmaceutical Industries in July last year.
Under the agreement, Menagene Pharmaceutical Industries will be acquiring the marketing authorization and own the exclusive rights to sell Nesbell in 6 countries in the Middle East, including Oman, Saudi Arabia, Chong Kun Dang starts supplying its finished Nesbell product to United Arab Emirates, Kuwait, Qatar, and Bahrain.
The drug is also increasing its presence in the Korean market.
According to the market research institution IQVIA, Nesbell’s sales last year amounted to ₩4.8 billion, which was a 150.9% YoY increase.
Its sales had only been ₩0.3 billion and ₩1.9 billion in the first year of its release and 2020, respectively.
Its sales has risen significantly last year to increase its market share in the same ingredient market to 18.5%.
Dong-A ST’s Nesp biosimilar ‘Darbepoetin-α’ is also slowly increasing its influence in the market.
After conducting a Phase 1 clinical trial on’ Darbepoetin-α,’ Dong-A ST signed a licensing-out agreement on the development and sale of its drug to Sanwa Kagaku Kenkyusho (SKK).
Based on a Phase III trial conducted in Japan to compare the efficacy and safety of ‘Darbepoetin-α’ to the original ‘Nesp,’ SKK received marketing approval from Japan's Ministry of Health, Labor and Welfare for the drug in September 2019 and launched the drug for sale in November of the same year.
Dong-A ST exports the finished products which were produced by DM Bio, a biosimilar company under Dong-A Socio Group, to SKK, after which SKK takes charge of its local sales.
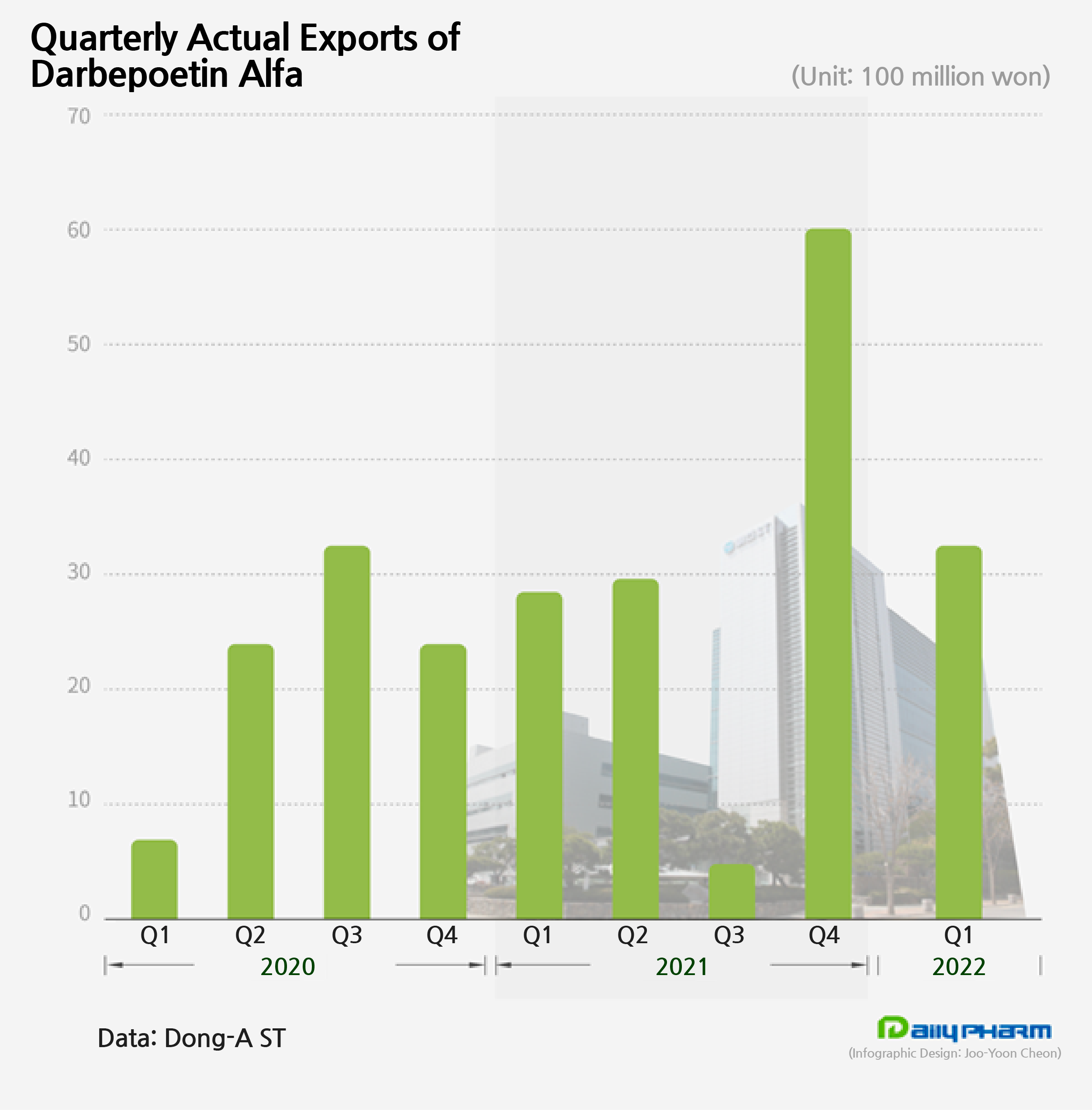
This is a decrease from the ₩6.1 billion made in Q4 last year, but still has been making ₩3 billion in sales every quarter.
Darbepoetin-α had first made ₩1 billion in the first year of its release, which increased to ₩8.8 in 2020.
Its sales then exceeded ₩10 billion in annual sales for the first time last year, recording ₩12.5 billion.
Darbepoetin-α’s cumulative sales totals at ₩25.6 billion.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









