- LOGIN
- MemberShip
- 2025-12-25 02:53:00
- 2nd P-CAB Fexclu to be released into the 800 bil market
- by Chon, Seung-Hyun | translator Alice Kang | 2022-06-30 05:52:40
Daewoong Pharmaceutical’s new drug, ‘Fexclu’ will be released next month.
The industry’s eyes are on whether the drug will raise a storm in the PPI class and P-CAB class antiulcer medication prescription market that is estimated to be worth over ₩800 billion.
The industry is also eyeing competition between Fexclu and K-CAB abroad.
◆Fexclu to be applied reimbursement from next month… prepares to target the ₩800 billion PPI·P-CAB market

With the reimbursement approval, the drug will be released in the market 6 months after it received approval on December 30th.
Fexclu’s insurance price cap was set at ₩939, which is 27.8% cheaper than its same-class drug K-CAB (₩1,300) Fexclu is a P-CAB class drug that treats gastroesophageal reflux disease (GERD).
The drug was approved for the indication of treating erosive gastroesophageal reflux disease (GERD).
P-CAB class antiulcer drugs competitively bind to the proton pump and potassium ion located in the final stage of acid secretion from the stomach wall to inhibit gastric acid secretion.
This class of drugs has quickly settled in the market since HK inno.N introduced its first P-CAB type of new drug ‘K-CAB’ in 2018.
Fexclu will be mainly targeting the PPI class and P-CAB class antiulcer drug market, which raised ₩842.1 billion in prescriptions last year.
The PPI class market made ₩732.5 billion and the P-CAB class market ₩109.6 billion last year.
The industry sees a high possibility of Fexclu’s success in the market.
K-CAB had already demonstrated the marketability of P-CAB class anticancer drugs during the past 3 years.
According to UBIST, K-CAB had raised ₩109.6 billion in outpatient prescriptions 3 years into its release last year, which is the first time a single brand of a homegrown novel drug had made annual prescriptions exceeding ₩100 billion.
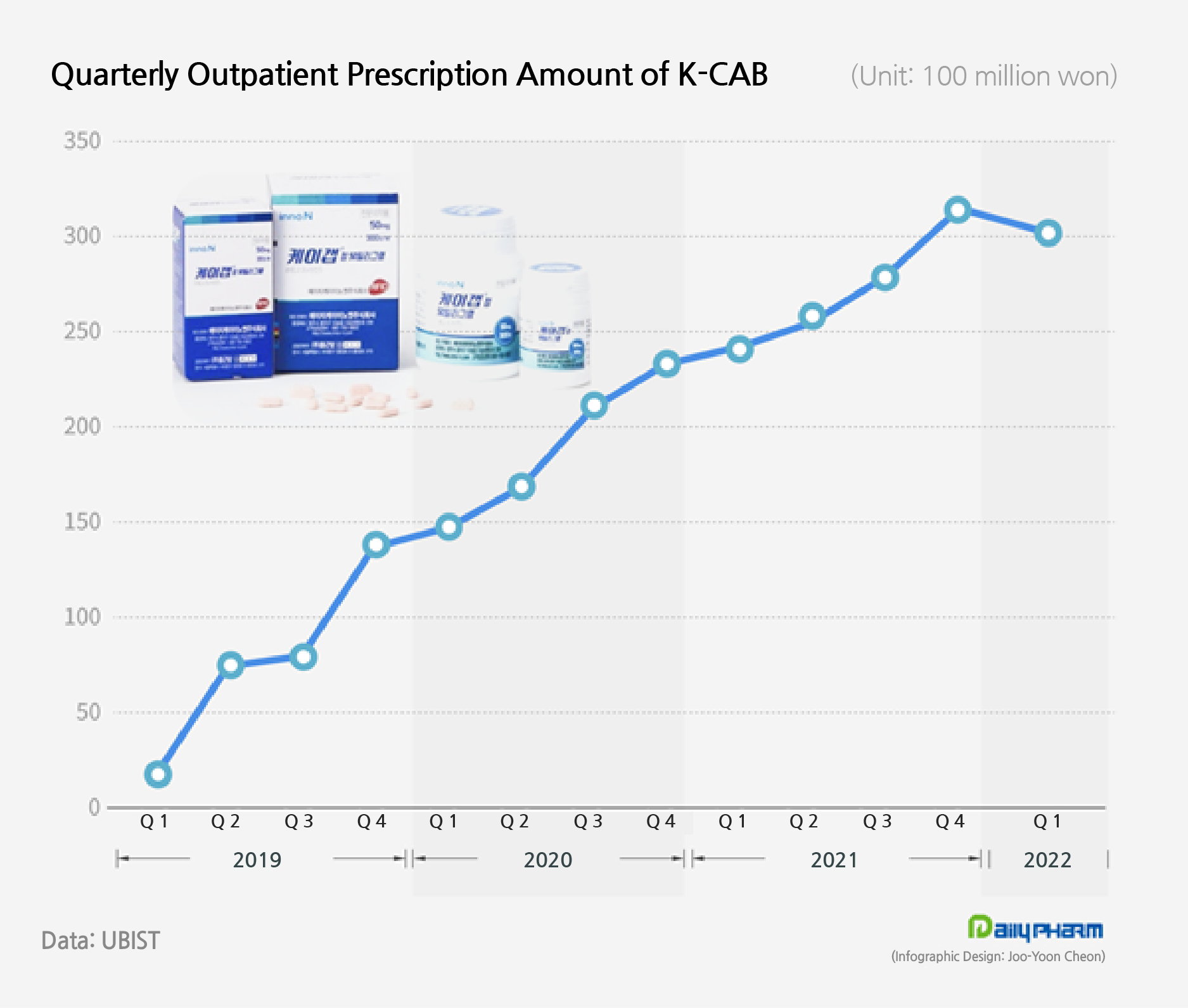
K-CAB’s sales continued to grow due to its advantages - having a faster onset of action than PPIs, is allowed to be taken regardless of meal ingestion, demonstrating superior symptom improvement, and showing longer-lasting night-time gastric acid suppression.
With HCPs and patients in the field already showing high satisfaction with P-CAB class antiulcer medications using K-CAB, Fexclu is expected to make a relatively easier way into the market.
Also, Fexclu’s insurance cap, which was set 30% lower than that of K-CAB may act as an advantage in the prescription market.
However, K-CAB owns a total of 4 indications, rendering it difficult for Fesclu to completely replace K-CAB in the market.
In addition to the treatment for erosive and non-erosive GERD, K-CAB sequentially secured additional indications as a combination therapy with an antibiotic for Helicobacter pylori eradication in patients with peptic ulcer or chronic atrophic gastritis.
Among the indications, insurance reimbursement is applied to K-CAB for the treatment of GERD and stomach ulcers.
Fexclu will also be targeting PPI-class drugs that account for the largest amount of the antiulcer drug market, as a significant amount of K-CAB prescriptions arise from patients who have switched to K-CAB from PPIs.
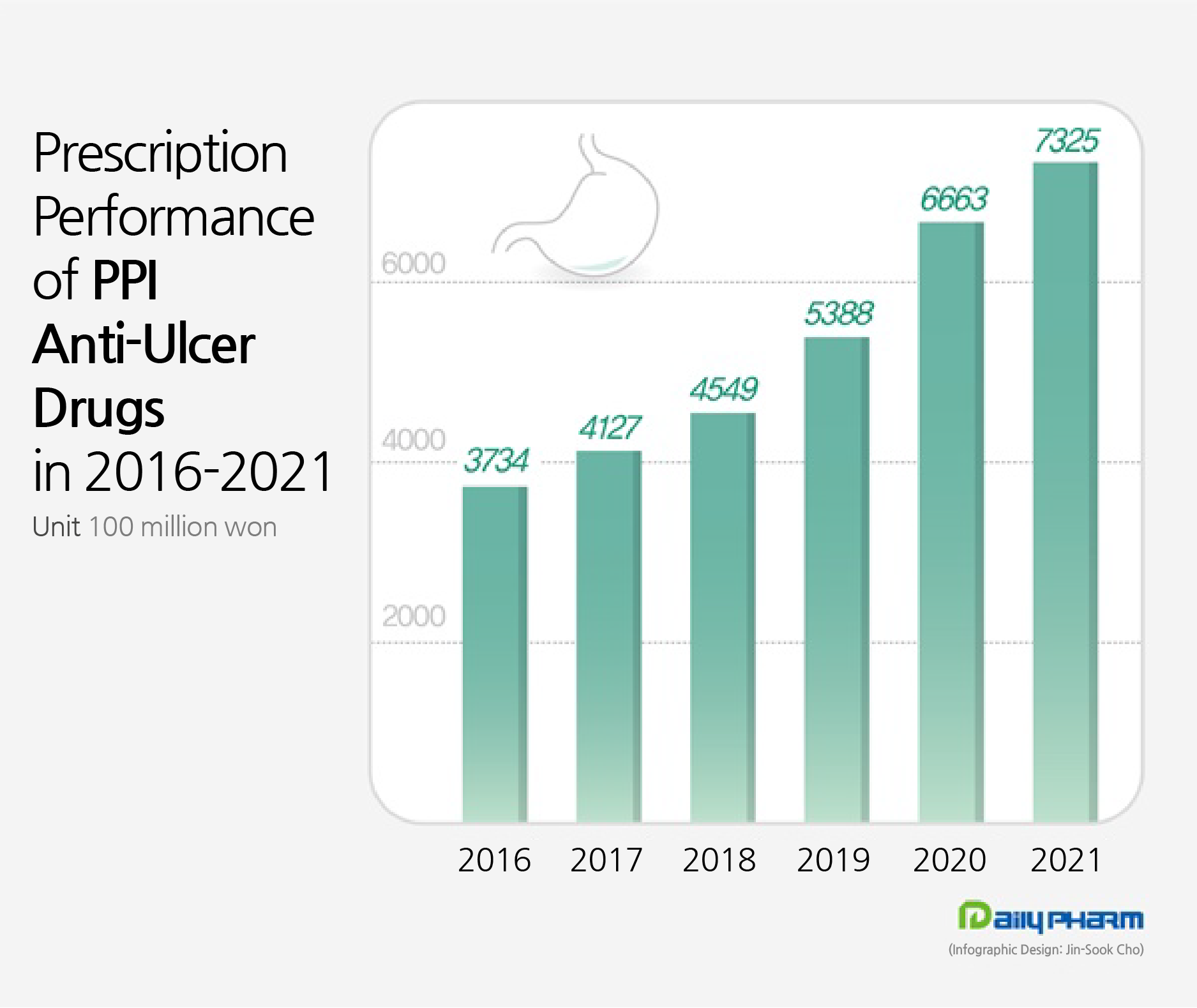
Last year, the amount of outpatient prescriptions for PPI class drugs recorded ₩732.5 billion.
This is a twofold increase in 5 years from the ₩372.4 billion in 2016 and is continuing to record high growth.
Daewoong Pharmaceuticals had signed 6 export agreements with 15 countries in North America, Latin America, China, Middle East to export Fexclu.
In 2020, the company signed a contract with the Mexican pharmaceutical company Moksha8 and Brazil's EMS for the local marketing and sales rights of Fexclu and paved the way for Fexclu’s entry into Central and South America.
In March last year, the company signed a $384.5 billion contract with China's Shanghai Haini Pharmaceutical.

company Neurogastrx and handed over the development, authorization, and sales rights of Fexclu in the US and Canada.
Through the agreement, Daewoong Pharmaceutical secured a 5% stake in Neurogastrx as an upfront, non-refundable fee, and was assured up to $430 million in development, regulatory, and sales milestone payments.
It then signed 2 additional export contracts to enter 10 countries in Central and South America and the Middle East.
In total, Daewoong secured up to ₩1.2 trillion through export agreements for Fexclu.
K-CAB has entered the market of a total of 34 countries through technology or finished product exports.
HK Inno.N had first signed a technology export agreement with the Chinese company Shandong Luoxin Pharmaceutical in 2015.
The deal was to receive $18.5 million in upfront payments and clinical development, regulatory, and sales milestone payments.
The company estimated that the agreement amount would rise to $95.29 million with royalties incurred after the local commercialization of K-CAB.
Also, HK InnoN signed an export agreement for the finished K-CAB product with the Mexican pharmaceutical company Laboratorios Carnot in February 2019 to export K-CAB to 17 countries in Central and South America.
Its export amount, including supply price, has amounted to $84 million over the past decade.
Exports agreements have been more actively made after K-CAB was released in Korea.
In September 2019, contracts were signed to supply the finished drugs to Indonesia, Thailand, and the Philippines, and in 2020, export agreements were made to export K-CAB to Mongolia and Singapore.
Last year, the company also signed export contracts with companies in Vietnam, Malaysia, the US, and Canada.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









