- LOGIN
- MemberShip
- 2025-12-25 01:06:51
- 5-year PDRN dispute comes to an end... PharmaResearch wins
- by Kim, Jin-Gu | translator Alice Kang | 2022-07-27 05:49:44

The industry expects the trial win to bring momentum to the rise of PDRN-based pharmaceuticals and devices, such as ‘Rejuran’ and ‘Conjuran.’ If the company also wins the patent infringement trial that follows, PharmaResearch will also be able to hold the generics in check, solidifying its position as the original product.
◆ Managing Director Kiseok Kang, “Court recognized our original technology" According to industry sources, the Patent Court of Korea ruled in favor of PharmaResearch in the reversed and remanded retrial on the PCRN manufacturing patent invalidation between PharmaResearch and BMI Korea on the 21st.
This brought an end to the 5-year long legal dispute that started in 2017.
In a phone interview with Dailpharm after the trial, Kiseok Kang, Managing Director of PharmaResearch, said, “After the long struggle, PharmaReserach was able to win the patent litigation and be recognized for our original technology.
We plan to actively make this known to the market and solidify our position as an original product.
He added, “With the final ruling made in the invalidation trial, the patent infringement suit that had been temporarily suspended due to the invalidation trial will be finalized soon.
We are reviewing various strategies from multiple directions on how to proceed in the future.” The remark reflects Kang’s intent to hold generics in check through the patent infringement suit while strengthening the marketing and sales power of the original product while seeking new product development.
◆Twist after twist…PharmaResearch finally wins the patent invalidation trial after 5 years PharmaResearch had long been in a legal dispute over the patent for the PDRN manufacturing technology with BMI Korea.
When BMI Korea received approval for ‘ HiDr injection,’ a same-ingredient latecomer drug in 2016, PharmaResearch pointed out that the drug’s API and manufacturing method were inappropriate.
As a naturally-derived ingredient, PDRN has a more complex manufacturing method than chemical drugs.
Therefore, PharmaResearch claimed that the difference in the origin of the raw material or manufacturing method can bring completely different efficacy and safety results, rendering BMI Korea’s drug completely different from its original.
Also, the company claimed patent infringement, pointing out that optimizing DNA fragments extracted from fish for human use is PharmaResearch’s proprietary technology that requires demanding quality and manufacturing process control and management.
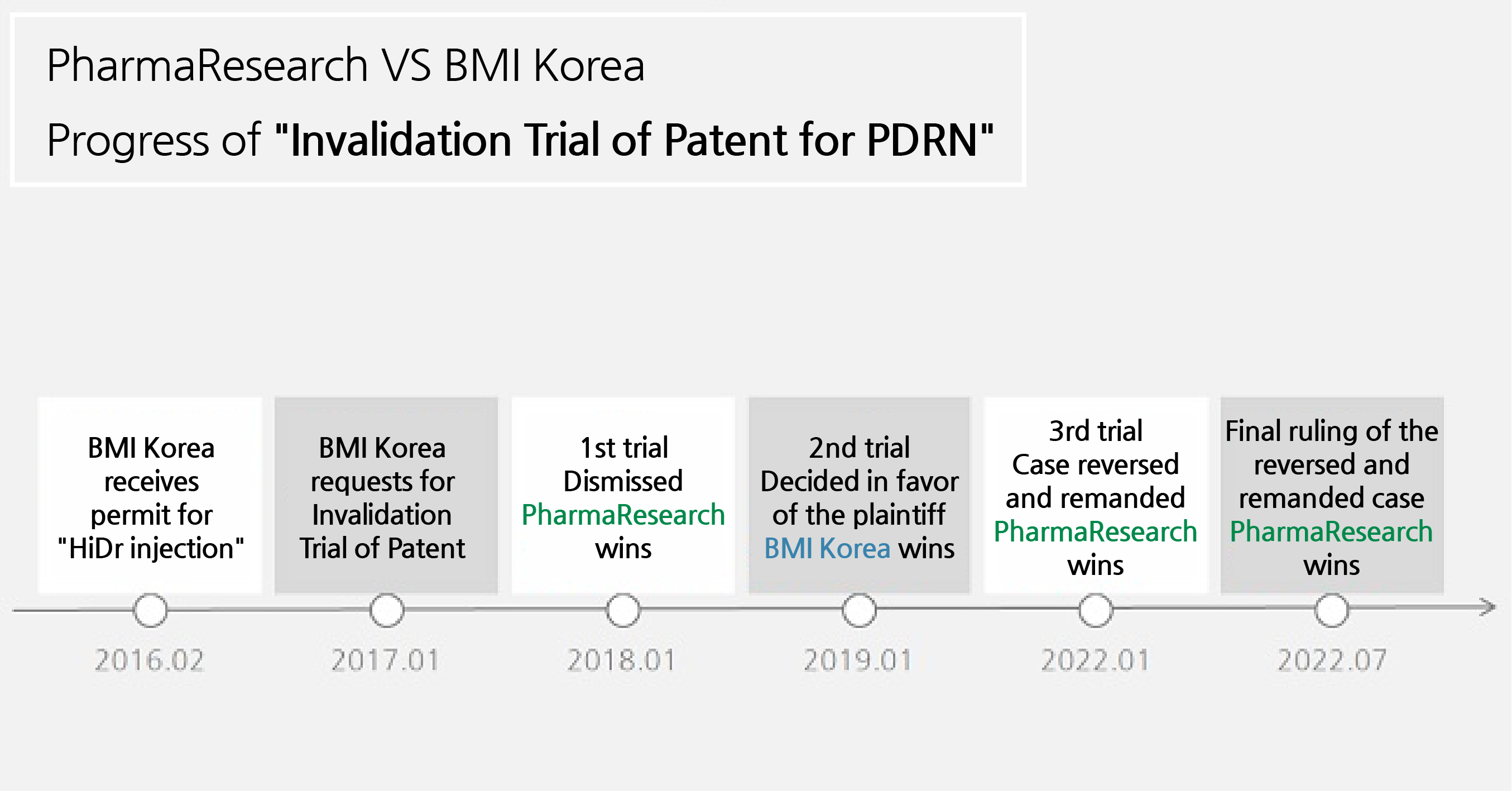
In January 2017, BMI Korea filed a trial against PharmaResearch to invalidate its PDRN manufacturing method.
The legal battle that ensued showed reversal after reversal.
PharmaResearch won the first trial with the Korean Intellectual Property Trial and Appeal Board rejecting BMI Korea's claim in January 2018.
In January 2019, the Patent Court of Korea overturned the first trial's ruling.
The court ruled that the PDRN patent was invalid.
Once again, the victorious smile dawned on the other side at the Supreme Court.
In January this year, two years after the hearing began, the court overturned the original ruling and decided to reverse and remand the trial.
Following the decision, the Patent Court of Korea made the final ruling on the 21st of this month in favor of PharmaReserach.
The 5-year long legal dispute has finally come to an end.
With the win, PharmaReserach is expected to establish its own area in the market for PDRN-based pharmaceuticals and medical devices market.
Currently, PDRN-based polydeoxyribonucleotide ingredient drugs come in two forms – pain treatment injections and eyedrops.
26 painkiller injections including PharmaReserach’s Placentex Inj and Rejuvenex Inj, and 10 eyedrops including the company’s Re-an Eye Drops are in competition in the market.
PharmaReserach plans to actively use the court’s ruling to market its two market leaders.
◆Interest rises for the patent infringement suit…may halt the entry of generics depending on its results Results of the patent infringement suit that is being tried separately against BMI Korea are also gaining much attention.
PharmaResearch, which owns the exclusive license for the PDRM manufacturing technology, had separately filed a ‘Damages Claim Based on a Patent Right Infringement (civil case)’ against BMI Korea in 2016, apart from the patent invalidation trial.
However, the claim had been turned down by the Seoul Central District Court.
Since then, the infringement suit had been temporarily suspended with the original suit continuing to the second and third trials.
If PharmaResearch wins this case as well, sales of HiDr will be prohibited.
From PharmaResearch’s view, this will allow the company to keep its biggest competition in check.
PDRN is a pharmaceutical product extracted from the reproductive cells of salmon or trout and is used for skin regeneration, such as wound healing and tissue repair, etc.
It selectively responds to act on damaged skin areas, reduce inflammation and regenerate tissue.
The official name of the patent is ‘Polynucleotide fragments complex separated from fish's semen or egg and its manufacture.
It is named ‘'DOT™(DNA Optimizing Technology).’ Its patentee is Mastelli in Italy, and PharmaResearch has the exclusive license for the patent in Korea.
A product made from polydeoxyribonucleotide sodium, which is extracted from salmon semen, was first released in Korea through PharmaResearrch’s Placentex Inj that was approved in 2008.
The company introduced the drug through a strategic partnership with Mastelli.
PharmaResearrch, which had been importing and selling Placentex in Korea, set out to localize PDRN.
Through joint research with KIST, the company developed a PDRN/PN extraction technology.
Since it established the technology to extract-separate-refine the active material from salmon returning to the eastern coast of Korea in 2012, the company has been manufacturing its own PDRN ever since.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









