- LOGIN
- MemberShip
- 2025-12-24 23:29:00
- Zolgensma, which is the same price as an apartment
- by Moon, sung-ho | translator Choi HeeYoung | 2022-09-08 05:58:35
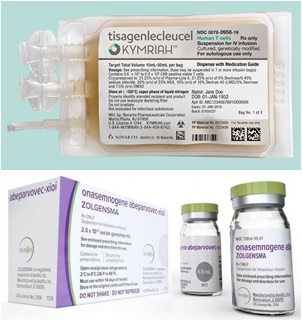
Since the treatment is so expensive, "risk" management issues such as damage and loss that may occur during administration are acting as a key issue.
#Kymriah, the CAR-T treatment of Novartis Korea, which entered the benefit range this year, and Zolgensma, the treatment of Spinal Muscular Atrophy (SMA), are representative.
According to the pharmaceutical industry and the medical community, Novartis Kymriah Korea has been applied as a health insurance benefit since April, and it has been confirmed that it has been administered not only at university hospitals in the Seoul metropolitan area but also at university hospitals in provincial areas.
Zolgensma, who has newly entered the health insurance benefit zone since August, has passed the Drug Committee (DC) of major Big 5 hospitals, including Seoul National University Hospital and Seoul Asan Hospital, and is preparing to administer patients in earnest.
As expensive treatments, which cost hundreds of millions of won alone, are administered in earnest at the clinical site, "risk management" that may occur is emerging as a key issue.
For example, the question is who will be responsible for the damage or loss of the treatment in the process of handling patients with expensive treatments.
This is because the price of the treatment is expensive, so both hospitals and pharmaceutical companies can be burdened depending on where they are responsible.
Moreover, in Korea, it is recognized as a bigger problem because there is no insurance system that can be called a safety device in preparation for the human risk of such expensive treatments.
However, there is a difference if Kymriah and Zolgensma are directly compared in terms of risk management.
First of all, Kymriah, which entered the benefit range, is a pair due to the nature of the treatment, that is, a system that prepares two treatments in case of an emergency.
An official from Novartis said, "In the case of Kymriah, due to the nature of the treatment, two treatments are prepared in case of an emergency." He explained the background, "As we make a treatment with T cells collected from the patient's blood, we can make two treatments with the same sauce." Kymriah reached up to 500 million won in the United States for a single administration, but in Korea, the patient's burden was lowered to up to 5.98 million won as it was set at 360.4 million won through drug price negotiations.
Against this background, Um Ki-sung, a professor of blood medicine at Seoul St.
Mary's Hospital, said, "In the process of introducing Kymriah, Novartis said that he would not receive drug costs if it was not actually administered, regardless of who did it wrong." Professor Um Ki-sung explained, "If a patient receives a lot of chemotherapy during the three-week administration, lymphocytes may not be extracted or cells may not come out." Professor Um said, "This means that pharmaceutical companies will not take issue with this and will only receive the drug price when the administration is completed." The problem is Zolgensma, which domestic drug price has been set at nearly 2 billion won.
This is because the characteristics are different from Kymriah itself and there is no insurance system to prepare for risks that may occur during the administration process.
In particular, in the case of Zolgensma, it is also worrisome that it differs from Kymriah in that the process is different, such as putting genes in vectors and transporting them so that they do not die.
For this reason, there are opinions among large hospitals that are concerned about this.
On top of that, Evrysdi, which is considering salaries as SMA treatments following Zolgensma, is also said to have to come up with a safety device for human risk.
In this regard, the HIRA, which is considering benefits, reportedly asked pharmaceutical company Roche to come up with a plan.
As Evrysdi is an oral drug, it means that safety devices should be prepared to prevent risk of patient loss.
This is because Evrysdi will not have a high drug price compared to Zolgensma and Spinraza, but it is widely expected that it will be set as a burdensome drug price for patients.
Novartis is in a position to discuss the plan with hospitals as it is scheduled to be administered in earnest according to the application of Zolgensma benefits.
"Since it is an expensive treatment, it is not easy for hospitals and pharmaceutical companies to pay for it," a Novartis official said.
"In the near future, Seoul National University Hospital is discussing how to manage risks and has been partially coordinated." "I think we will need to continue discussions in the future," he explained.
He said, "I checked the insurance company in terms of human risk safety, but there is no insurance company that covers it." He added, "There is no internal determination of responsibility at the moment, but since it is the first time, we have to continue to discuss it in the future.".
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









