- LOGIN
- MemberShip
- 2025-12-24 13:47:49
- Concerns over Leclaza's sluggish secondary indicators
- by | translator Choi HeeYoung | 2022-12-06 05:57:26

We cannot rule out the possibility that a similar situation to Tagrisso, whose effectiveness was questioned by the Asian OS, will be reproduced.
The global clinical director (PI) explains that it is not easy to secure statistical significance due to the relatively high ratio of crossover converted to third-generation treatments due to confirmed resistance mutations among control patients.
Cho Byung-chul, a professor of oncology at Yonsei Severance Hospital, who oversees LASER301 clinical trials, said at a press conference held in Singapore on the 3rd (local time), "Leclaza failed to meet the statistical significance of OS improvement because there was a high crossover rate of about 40 percent," adding, "There is no room for resistance change now."

The first evaluation index was the progression-free survival period (PFS), and Leclaza recorded a 20.6-month PFS, which reduced the risk of disease progression and death by 55% compared to the control group, demonstrating high improvement.
OS, the secondary indicator, has 29% data maturity, and sufficient data has not been collected.
However, the survival rate of Leclaza at 18 months after administration was not statistically significant compared to the control group (p=0.116).
At the time of 29% of data collection, 25% (49) of the Leclaza group died, and 32% (64) of the control group died.
Looking at the trend of the graph of the total survival period released on this day, the survival rate of the Leclaza group and the control group becomes almost similar from 27 months after administration.
The final OS data will be released at the end of next year.
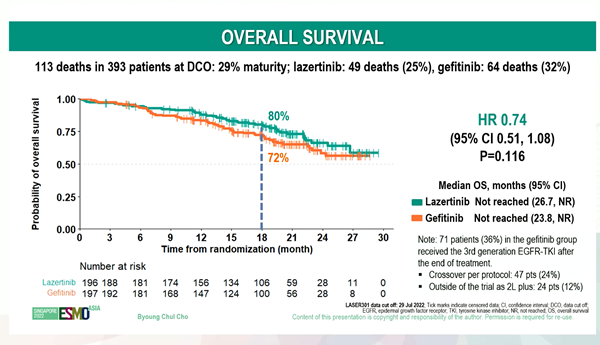
(Source: ESMO) Professor Cho explained that the high rate of crossover in the control group that received the first-generation treatment affected Leclaza's OS.
Crossover refers to allowing patients classified as control groups to take other treatments at an ethical level if they do not see treatment effects due to resistance during the administration of control drugs.
In phase 3 clinical trials, 24% (47) of 197 control groups changed the treatment to Leclaza through crossover.
12% (24) stopped clinical trials and administered other treatments.
A total of 71 people (36%) stopped administering control drugs and switched to third-generation treatments.
Tagrisso, the first 3rd generation EGFR formulation conducted earlier, was also affected by the OS by allowing crossover at the time of phase 3 clinical trials.
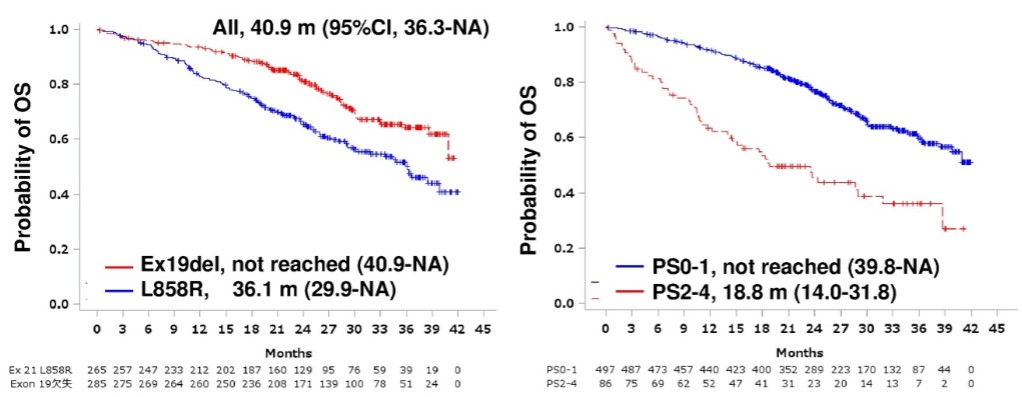
At that time, 85 (31%) of 277 patients in the clinical control group stopped administering first and second-generation treatments and received Tagrisso as a follow-up treatment, which caused "data bias." Because of this, Tagrisso's effectiveness was questioned by Asians.
Concerns have been raised that Tagrisso's OS benefits are unclear in Asians.
Tagrisso has also been pointed out as the main reason why it has not been listed on the domestic primary benefit so far.
The effectiveness of Tagrisso is being proven in clinical practice.
According to the recently released Real World data in Japan, Tagrisso recorded an OS of 40.9 months for more than three years.
About 90% of EGFR-mutated non-small cell lung cancer patients in the United States and Europe as well as Japan are being treated with Tagrisso in the first round.
Leclaza was also influenced by the clinical environment where the diagnosis of T790M mutations, the first and second generations of resistant mutations, became active.
Professor Cho said, "At the time of phase 3 of Tagrisso, the T790M mutation itself was unfamiliar.
Naturally, the diagnosis was not active.
However, now, follow-up treatments have emerged and T790M tests are being actively conducted.
Because of this, nearly 40% of patients were crossover.
In other words, as resistance diagnosis was actively conducted, more patients were found to receive follow-up treatment.
He added, "Leclaza's higher OS effect was observed when analyzed by applying the IPCW (Inverse Probability of Sensing Weight) technique." The IPCW technique refers to an analysis that minimizes "bias" by correcting crossover patient data.
Professor Cho said, "I don't think the failure of OS to secure statistical significance will lead to concerns over the Leclaza effect." Ross A.
Soo, a professor at the National Cancer Center in Singapore, also said, "It is still too early to evaluate OS, and the effectiveness of individual drugs can be confirmed by PFS indicators, and OS is an indicator of the entire treatment, including follow-up treatment."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









