- LOGIN
- MemberShip
- 2025-12-24 07:27:42
- Why no news is being heard on reimb extension for Bavencio
- by | translator Alice Kang | 2022-12-28 05:48:31
Merck and Pfizer’s cancer immunotherapy ‘Bavencio (avelumab)’ is having trouble expanding its reimbursement in Korea.
Although the agenda has passed deliberations by the Cancer Disease Deliberation Committee (CDDC), the follow-up process was sluggish and no progress has been made for 8 months.
According to the pharmaceutical industry on the 27th, the indication expansion agenda passed the Health Insurance Reimbursement and Assessment Service's CDDC deliberations in April, after which no news is being heard on its progress.
The agenda was at a standstill because it was not presented for deliberation by the Drug Reimbursement Evaluation Committee (DREC).
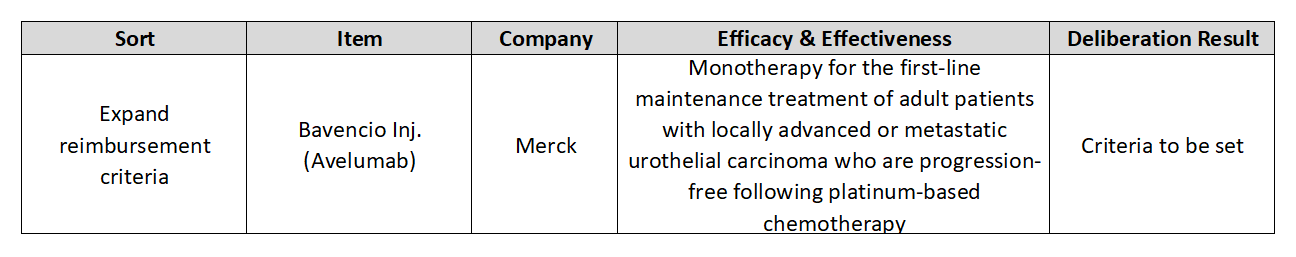
This was when the reimbursement standards were set for Bavencio as first-line maintenance monotherapy for adult patients with locally advanced or metastatic urothelial carcinoma.
However, its reimbursement for the bladder cancer indication is still far off.
This is because DREC has not deliberated on expanding the coverage for Bavencio for the past 8 months with no hard plan set for its deliberation in the future.
Bavencio is not the only drug that has seen little progress after passing CDDC.
Ono Pharmaceutical’s ‘Bratovi’ passed CDDC meetings in January and received reimbursement standards in colorectal cancer, but has not been deliberated by DREC for a year since.
AstraZeneca’s ‘Lynparza’ that was set reimbursement standards for the treatment of BRCA-mutated prostate cancer, To list or expand the indication of anticancer drugs for reimbursement, the agenda needs to pass CDDC deliberations and then undergo reimbursement adequacy review by DREC, drug pricing negotiations with the NHIS, and finally pass the Ministry of Health and Welfare’s Health Insurance Policy Deliberative Committee deliberations.
In general, the statutory processing period for drug reimbursement evaluations conducted by HIRA's CDDC and DREC is set at 120 days (150 days for RSA drugs).
However, with reasons such as requests for supplementary data, the statutory review period has not been properly observed until now.
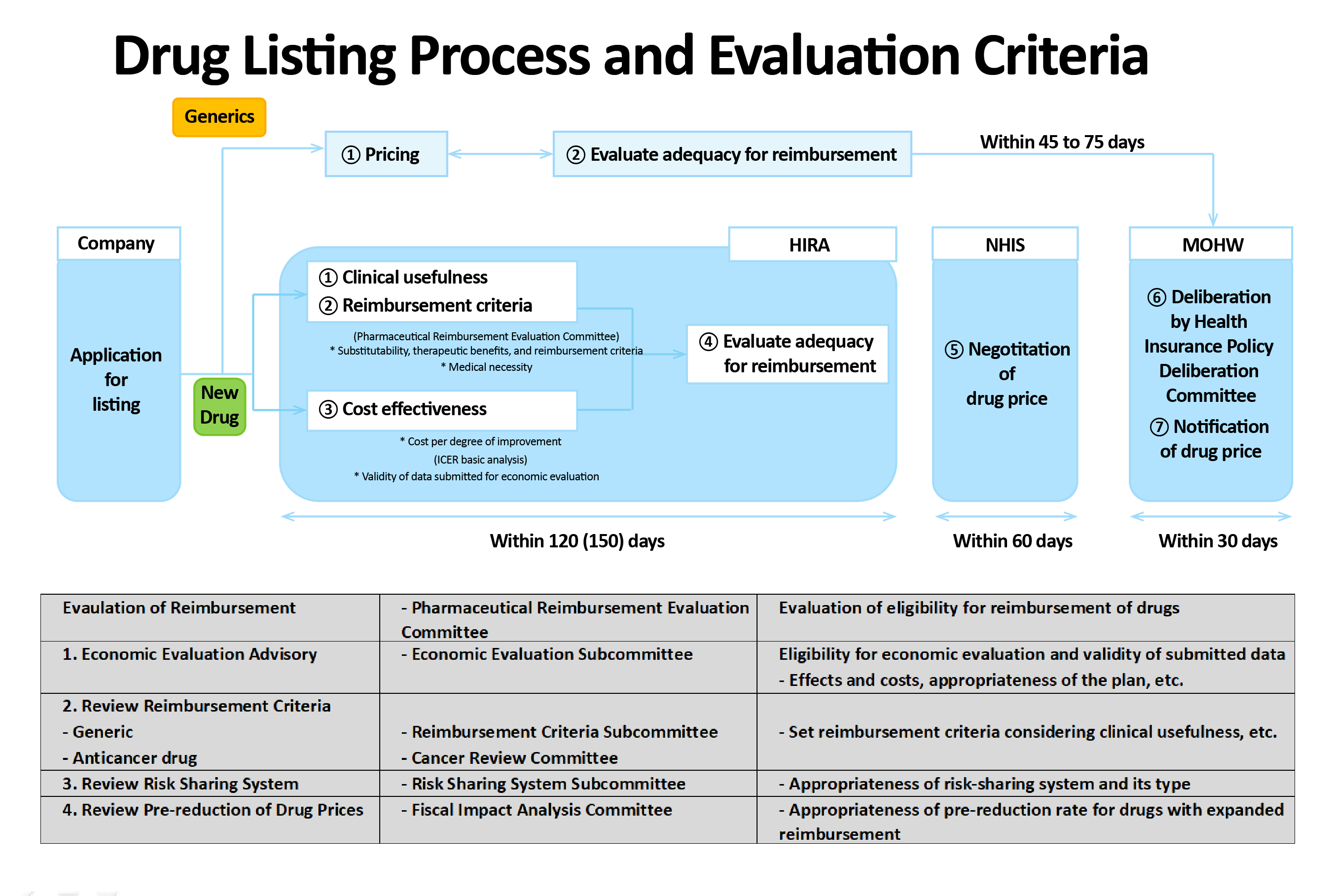
Also, with the rise of the COVID-19 pandemic and the introduction of high-priced new drugs, fiscal soundness has emerged as an important issue.
This is why more and more reimbursement expansion deliberations for existing drugs are falling behind in priority.
Bavencio’s indication as maintenance therapy that the company is attempting to expand reimbursement to is a sort of 1.5-line therapy used in patients with urothelial carcinoma who have not progressed after using standard chemotherapy.
In the Phase III JAVELIN Bladder 100 trial, the median overall survival (OS) was extended by over 7 months for patients who received Bavencio+BSC compared to best supportive care (BSC) care alone and reduced the risk of death by 31%.
The 1-year overall survival rate was 71.4% in the Bavencio group, higher than the 58.4% observed in the comparator group.
The delay in DREC’s deliberation of Bavencio for reimbursement as maintenance therapy can also be partially attributed to the existence of other cancer immunotherapies.
Currently, other immunotherapies including Keytruda, Tecentriq, and Opdivo also own bladder cancer indications.
The specific indications approved may differ for each drug.
Keytruda and Tecentriq are used in the first line.
However, only patients that are PD-L1 positive and who are not eligible for cisplatin-containing chemotherapy are allowed to use the drugs.
In the case of Opdivo, the drug is used as a second-line treatment in patients whose disease has progressed after chemotherapy treatment.
Keytruda and Tecentiq can also be used in the second line.
Tecentriq, which had been the only drug approved for reimbursement in the second line, was unable to meet the conditional approval requirements set by the MFDS and was removed from the reimbursement list in September, and Keytruda emerged in its place.
Tecentriq withdrew all bladder cancer indications after failing to demonstrate an effect for cancer in a Phase III trial.
The company’s actions are also expected to affect its approved indications in Korea.
As a result, the only reimbursed cancer immunotherapy option is Keytruda in the second line.
The industry sees the availability of a reimbursed immunotherapy option, although in the second-line, and the lack of a second-line option after using Bavencio as maintenance therapy as the barriers that interfere with the reimbursement expansion of Bavencio, as immunotherapies cannot be used in later-line therapies after failing treatment with such in previous lines of treatment.
However, this means that patients have to wait until disease progression to use immunotherapies in the second line.
And not all patients can use immunotherapies with disease progression.
Therefore, the opinion has been raised on the need for earlier use of immunotherapies.
Professor Se Hoon Park, Division of Hematology/Oncology at Samsung Medical Center, said, “Due to the limited amount of second-line treatment options for bladder cancer, it could be so far as said that no treatment exists for bladder cancer in the second-line, and we hope that the reimbursement adequacy evaluation for Bavencio will soon progress further based on scientific evidence.” Park said, “If a treatment can safely provide clinically significant effect over existing treatments, its reimbursement adequacy should be evaluated under the same standards regardless of whether the treatment is being used for other cancer types or not.” JinYoung Paik, President of the Korea Kidney Cancer Association, said, “Many bladder cancer patients have been asking about Bavencio’s reimbursement due to the lack of other effective treatment options after receiving first-line treatment with chemotherapy.
Bavencio is recommended as standard treatment in overseas guidelines and many significant data have been presented on the drug, but many patients cannot use it for economic reasons.
I hope reimbursement discussions on Bavencio be progressed soon.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









