- LOGIN
- MemberShip
- 2025-12-24 07:23:25
- Curing terminal cancer without treatment options
- by | translator Alice Kang | 2023-01-17 06:02:48

A, a 60-year-old Korean male patient, was diagnosed with gastric cancer several years ago.
After struggling to treat the disease for several years, the hospital told A that there was no other treatment option available for him and that he may not be able to live for more than 6 months.
As a last resort, A registered to enroll in the KOSMOS study and was told there was a breast cancer treatment available for the genetic mutation he was found with during genetic testing.
The breast cancer drug stayed effective for over a year, and after seeing a significant reduction in tumor size, A left his hospital bed and was able to receive the outpatient treatment he had only dreamed of.
The stage has been set to create a Korean-style precision medical ecosystem that can provide personalized treatment opportunities for cancer patients with terminal cancer who no longer have treatment options like Mr.
A.
A large-scale project involving academic societies, hospitals, research institutes including the Korean Society of Medical Oncology, Korean Cancer Study Group, Korea Health Industry Development Institute, National Cancer Center, Roche Korea, and Lunit Inc.
is set to start this year.
◆Use without indication or approval ’OK’…brings new hope to terminal cancer patients The clinical project, KOSMOS2(KOrean precision medicine networking group Study of MOlecular profiling guided therapy based genomic alterations in advance Solid tumors II), will be recruiting 1,000 patients for 3 years.
Subject patients are those with terminal-stage cancers that have a life expectancy of 6 months or less.
In general, these patients are advised to leave the hospital because there is no other drug available for their use in the hospital.
Therefore, their caregivers travel from hospital to hospital in search of one that can take care of their beloved’s illness just a little more.
When left with no other option, most of them end up receiving hospice care.
Enrolling in the KOSMOS2 study could open up new opportunities for these patients.
First, patients who enroll in the KOSMOS2 study receive next-generation sequencing (NGS) to check for genetic mutations.
Roche Korea provides an NGS-based comprehensive genomic profiling (CGP) Foundation Medicine Service.
The results are stored on ‘Navify Tumorboard,’ Roche Diagnostic Korea’s multidisciplinary tumor board (MTBs) that was developed specifically for cancer treatment.
It is a multicenter MTB that has been first attempted in the KOSMOS project that allows associated companies to share data.
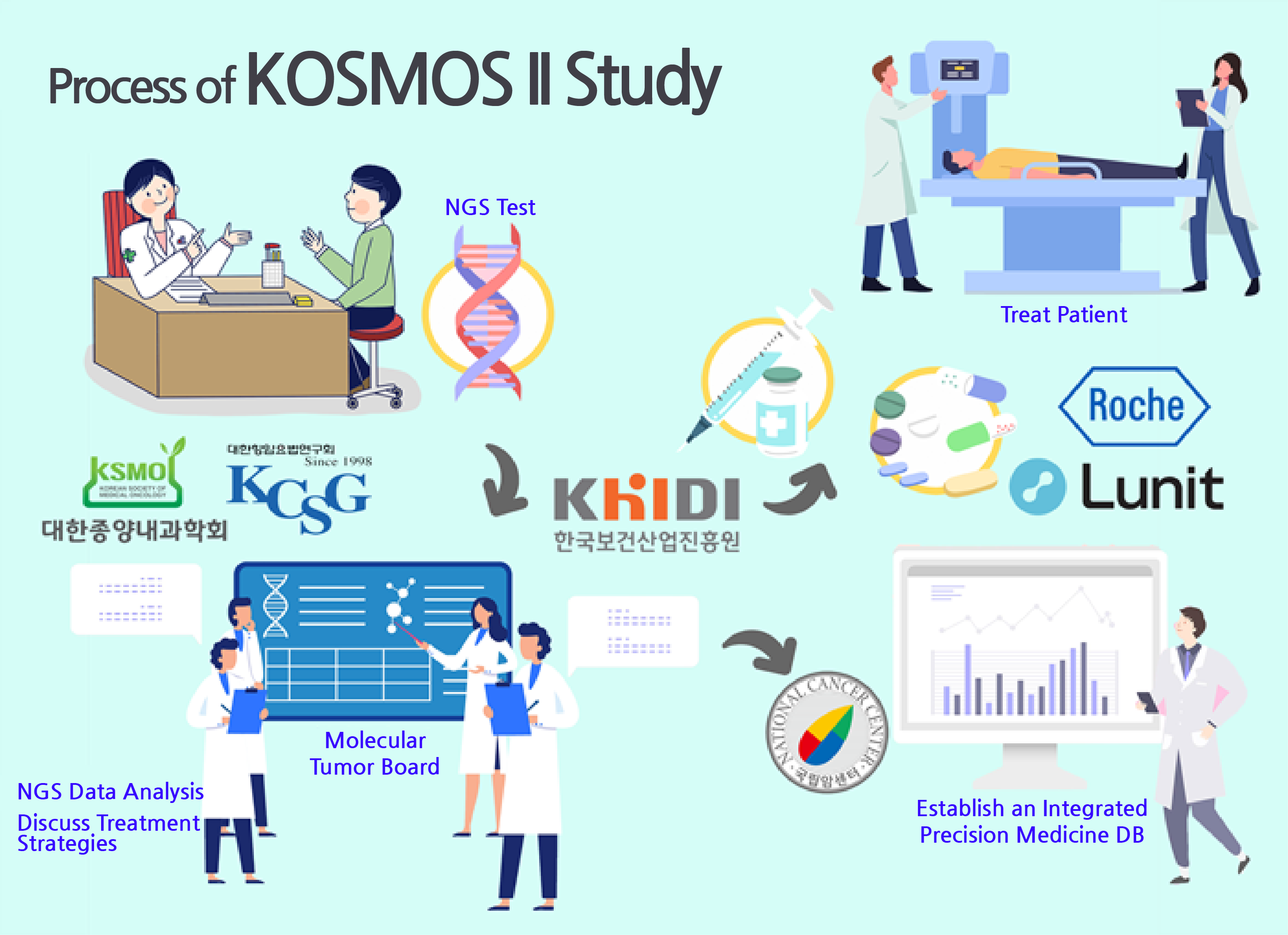
In the KOSMOS2 study, patients can attempt to be treated with drugs that are not approved in Korea or has no indication for their cancer if the doctors deem the drug appropriate.
Therefore, the patient can receive treatment with a new drug that is not normally available, and the drug cost is borne by the pharmaceutical company.
Of course, not all patients can receive treatment with new drugs.
A drug that targets the patient’s mutation that had been identified through genetic testing has to first exist.
The academic society estimated that around 30% of the patients will be able to receive treatment with new drugs.
However, in the KOSMOS1 study, which became the basis for the KOSMOS2 study, more patients than expected were provided treatment opportunities.
This was an unexpected achievement of precision medicine that even surprised the specialists.
◆Organic cooperation for Korean precision medicine...established a system for a virtuous cycle In the KOSMOS2 study, academic societies, research institutes, hospitals, and pharmaceutical companies organically work together to bring out the best results for each patient.
The Korean Society of Medical Oncology and the Korean Cancer Study Group will lead the study, developing the specific design for the study and generating clinical and genomic data.
As the Ministry of Food and Drug Safety’s designated national cancer data center, the National Cancer Center will be providing the technology and infrastructure required to build an integrated database for precision medicine.
The NCC will also select and classify the collected data to provide a high-quality DB.
Roche Korea will support pharmaceuticals and the optimal precision medical platform.
To promote the project, the Korea Health Industry Development Institute will connect institutions, domestic and foreign pharmaceutical companies, and genome and software companies for collaboration.
The first patient was recently enrolled in the KOSMOS2 study, which has been recruiting patients since the second half of last year.
The study will enroll patients for 2 years, follow up for 1 year, and conclude the study after 3 years.
The KOSMOS2 research project will provide patients with new treatment opportunities, and enable academia to realize precision medicine in the true sense regardless of their affiliation or region.
Research institutes and industries can use high-quality genetic DB of cancer patients to conduct new research such as new drug development.
In other words, a virtuous cycle of precision medicine and innovative new drug development will be created.
Yong-Woo Kim, Lead of the Biopharmaceutical Industry Team at KHIDI, said, “It is important to build an integrated clinical genomic database as the basis for precision medicine and new drug development of pharma and bio companies in Korea.
The organic cooperation between the government, academia, hospitals, and companies, will not only bring destructive innovation in precision medicine, but it will also mark the starting point for the revitalization of precision medicine in Korea."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









