- LOGIN
- MemberShip
- 2025-12-24 07:23:26
- Practice manual issued for COVID-19 treatments in Korea
- by | translator Kim, Jung-Ju | 2023-01-30 05:54:38
Doctors have issued a ‘Practice Manual’ to provide an understanding of how to use the COVID-19 treatment in one glance.
The manual was prepared to directly address difficulties HCPs experienced prescribing COVID-19 treatments in the field due to a large number of contraindicated drugs.
The Korean Society for Antimicrobial Therapy (KSAT) recently distributed a manual for the actual prescription of Paxlovid, Pfizer's oral COVID-19 treatment.
The manual was designed to allow easier understanding for HCPs on whether to refrain prescription of Paxlovid or make dosage adjustments according to a patient's medication status.
Paxlovid is the first oral COVID-19 treatment that was approved in Korea.
The drug was granted Emergency Use Authorization in December 2021 and was indicated for moderate-to-severe COVID-19 patients at high risk of progressing to severe disease.
At the time the authorities initiated the supply of Paxlovid, the authorities had also issued treatment prescription guidelines, however, the guidelines issued then were more than 100 pages long and were not easy to use in the field.

The KSAT collected the main areas of consideration of up to 100 drugs that require attention so that they could be applied to practice right away.
The manual summarized the cautions that do not appear in the Drug Utilization Review (DUR) program.
The guidelines also contain, a list of over-the-counter drug products that contain ingredients that require special attention.
In an interview with Daily Pharm, Eun-Joo Choo, Director of the Insurance Committee at KSAT and Professor of Soonchunhyang University College of Medicine, who contributed to the preparation of the guidelines for Paxlovid, said, “The existing guidelines were too detailed to identify what precautions apply for each patient's medication.
We hope that the guideline can contribute to increasing the practical prescription rate of oral COVID-19 treatments that lower disease severity rate,” stressing the importance of prescribing the COVID-19 treatment.
The full interview transcript of Daily Pharm’s interview with Professor Choo is as follows.
- A Paxlovid prescription guideline that the government issued already exists.
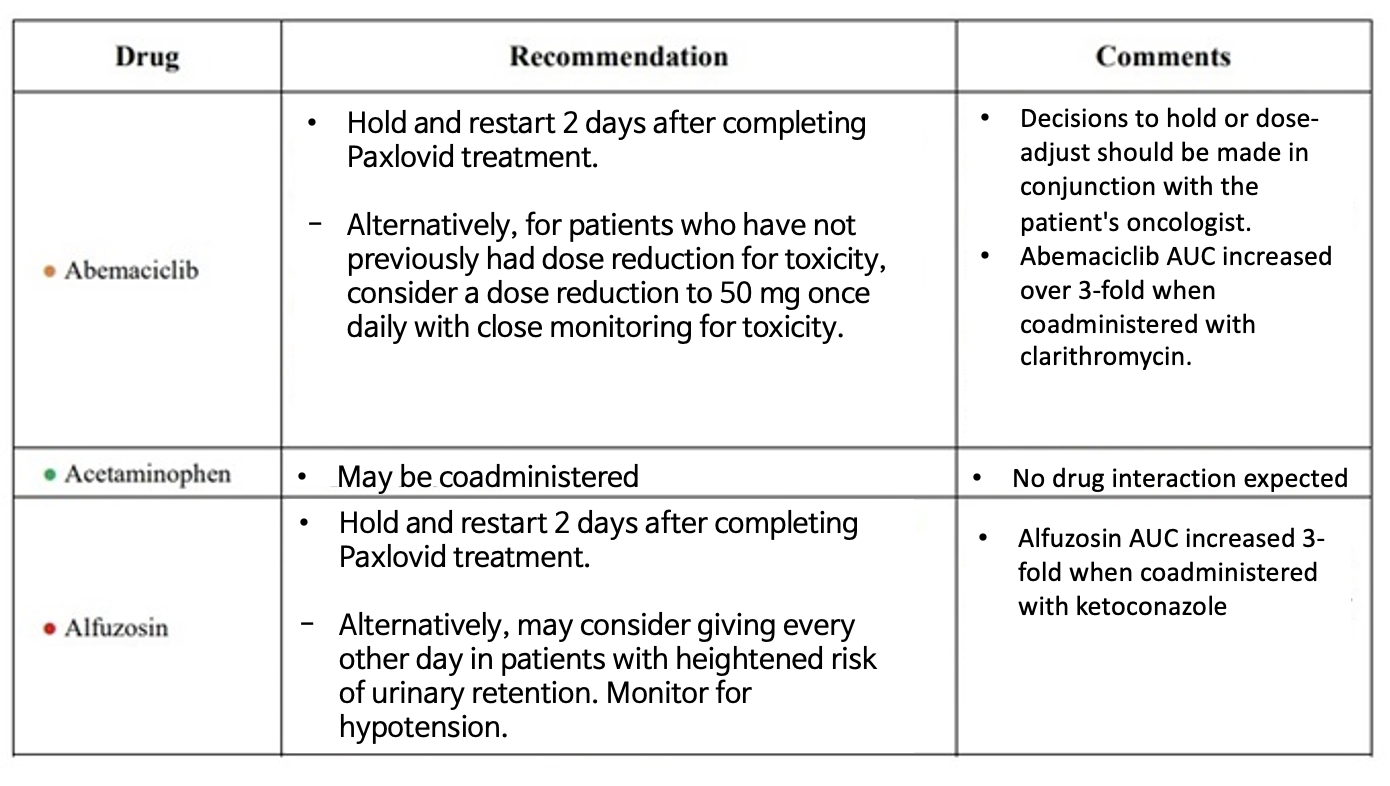
Why did KSAT see a need to prepare a separate guideline?? =KSAT’s practice guideline is more of a ‘practical manual' that allows healthcare professionals to understand contraindicated drugs and dose adjustment at a glance in the field during prescriptions.
The Paxlovid prescription guidelines issued by the government are over 100 pages long.
It contains too much detail for HCPs to immediately absorb and apply in the field.
This is why HCPs had difficulty prescribing the drug in the field, being an unfamiliar drug that is difficult to use.
John’s Wort’ substances that are contraindicated, ▲drugs that require use with caution when co-administered, etc.
In particular, the drug substances that require caution in the use and key recommendations were summarized in a table format so that they can be applied immediately in practice.
-I first thought the contraindicated drugs will be easy to identify as they immediately show up in the DUR database, but it seems that I was wrong.
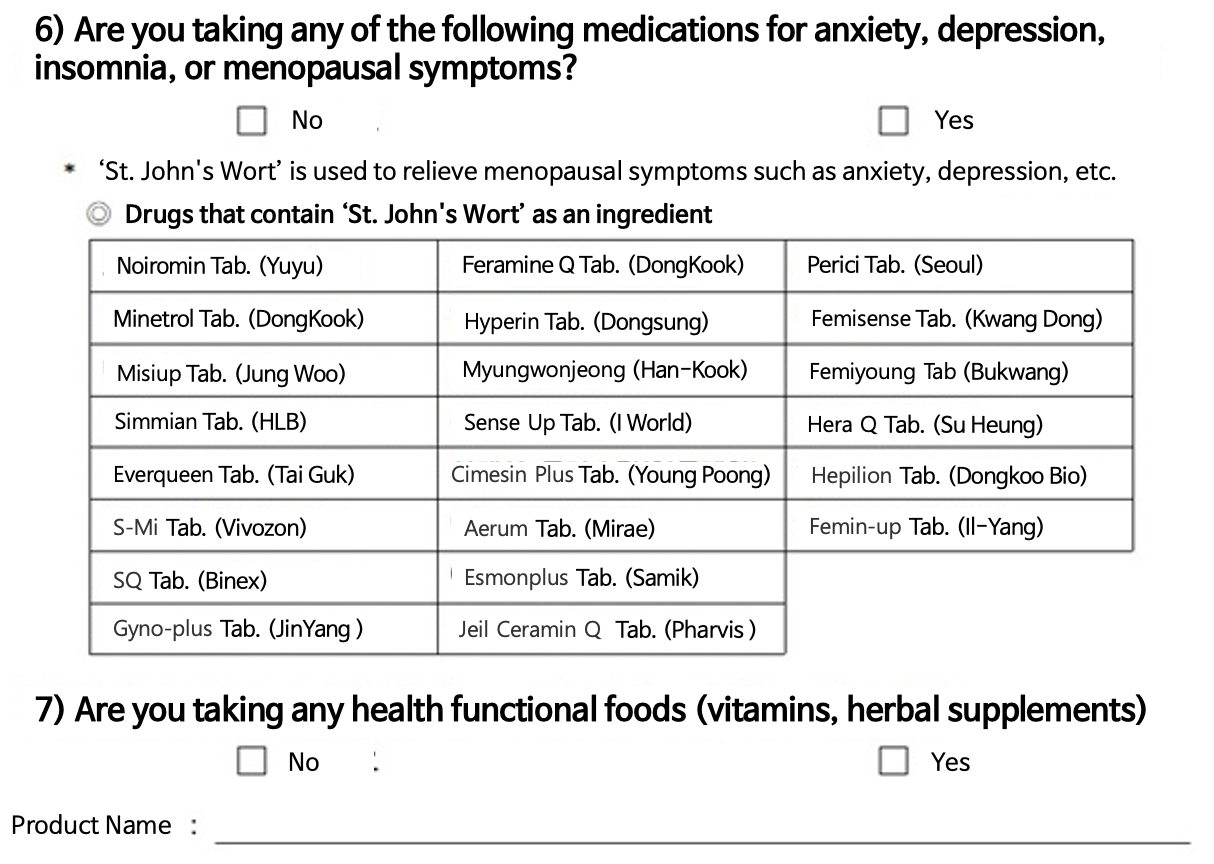
In particular, it seems that it would have been difficult to identify all the products that contain St.
John’s Wort, as the substance is commonly used in OTC drugs and health functional foods. =That’s right.
Only the 7 contraindicated drugs that must not be prescribed together pop up on the DUR, which includes epilepsy substances such as St.
John's Wort.
Substances commonly used by patients for hyperlipidemia, heart failure, gout, etc., that can be temporarily discontinued or substituted to administer Paxlovid, but such information is not reflected in the DUR.
In addition, there are about 100 drugs that require attention, such as dose reductions, etc.
It's not easy for doctors to know all this during prescriptions.
In particular, the St.
John's Wort substance is contraindicated, regardless of whether it is a prescription drug, general OTC drug, or health functional food.
Since doctors need to check directly with the patient on whether or not they use the substance, a list of the OTC drugs was prepared that specifies the product and company names.
The self-checklist also asks patients once more to confirm whether they are taking OTCs or health functional foods.
Fortunately, St.
John's Wort is mainly used in women, but the rate of those taking it at the age of over 60 is not that high.
What do you believe is the reason behind this? = During our webinar, one of the most common concerns that the doctors had was ‘Whether they should use COVID-19 treatments in mild COVID-19 patients with few symptoms.’ Being a drug that the doctor hasn’t used before, in addition to the fact that the drug has many drug-drug interactions, and the treatments for COVID-19 are mainly conducted non-face-to-face, it is natural that doctors may be hesitant in using such unfamiliar drugs.
The part I want to emphasize is that oral COVID-19 treatments prevent patients with mild symptoms from progressing to severe diseases.
Patients may have minor symptoms at first, but if they do not take the right medicine at the right period, their disease can progress to severe disease, in particular in patients that are over the age of 60 or have underlying diseases.
In fact, quite a lot of inpatients who had COVID-19 were sent to the intensive care unit when there were no available vaccines or treatments.
However, this number decreased significantly after the introduction of COVID-19 vaccines and oral treatments.
Real-world data also showed that the rate of progressing to severe disease was reduced by about 50% with the prescription of Paxlovid.
I believe prescriptions will naturally increase after such data on Paxlovid’s actual effect and HCP prescriptions increase and accumulate.
-The majority of Korean people have been vaccinated several times.
Also, with so many having a history of COVID-19 infections, not many are concerned about reinfections.
Do you believe these people also should receive vaccinations with booster shots?
If they are high-risk groups, would they need to be prescribed oral COVID-19 treatments when reinfected?
If you look at the government’s reinfection rate data, the age-standardized risk of death in case of reinfection is 1.58 times higher than that at initial infection.
Just as people receive vaccination for the flu every year and then use Tamiflu when infected, COVID-19 also requires regular vaccination and treatment.
In fact, COVID-19 requires more caution as it has a higher rate of complications and disease progression than the flu.
Patients who have pneumonia due to COVID-19 suffer complications such as difficulty in breathing due to pulmonary fibrosis even after completing treatment.
Some people describe COVID-19 as a 'highly contagious cold', but I do not agree, as no one dies of a simple cold.
Also, the experience of the patient that receives the treatment is as important as the HCP that prescribes Paxlovid.
There is little positive feedback yet because not many patients have experienced the treatment.
However, the treatment has been proven to be effective, and in particular, it is more beneficial for patients that opt for at-home treatments.
Because of the definite benefits, I hope that patients do not worry too much and take their prescribed oral COVID-19 treatment as indicated.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









