- LOGIN
- MemberShip
- 2025-12-24 03:49:09
- Mavyret occupies 85% of HCV market...sales fall 36% in 3 yrs
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-02-06 05:51:14

Last year the drug increased its market share to 85%.
Despite this increase in market share, the drug’s prescription performance fell 36% over the past 3 years.
The analysis is that the absolute size of the Hepatitis C treatment market, which has a limited number of patients, has been decreasing due to the near-cure effect of the treatments in the market, which eventually led to a reduction in the overall market size.
◆Mavyret occupies 85% of the HCV market...prescription performance drops 36% in 3 years According to the market research institution UBIST on the 4th, Abbvie’s oral HCV treatment Maryret’s outpatient prescriptions recorded KRW 29 billion last year.
This was a 10% increase compared to 2021 and is considered to be due to Maryret’s scope of reimbursement being expanded from adults to adolescents aged 12 years or older.
With the reimbursement extension, its market share increased to 85%.
Mavyret had quickly expanded its influence in the market since its release in September 2018, with its benefits of being pan-genotypic and short treatment period.
In 2019, its market share increased to 70% and then to 75% in 2020 and 2021.
However, in the long term, the reduction in prescription performance is clear.
Outpatient prescriptions fell from KRW 45.6 billion in 2019 to KRW 35.7 billion in 2020, then to KRW 26.3 billion in 2021.
This amounted to a 36% decrease in prescription performance compared to 2019.
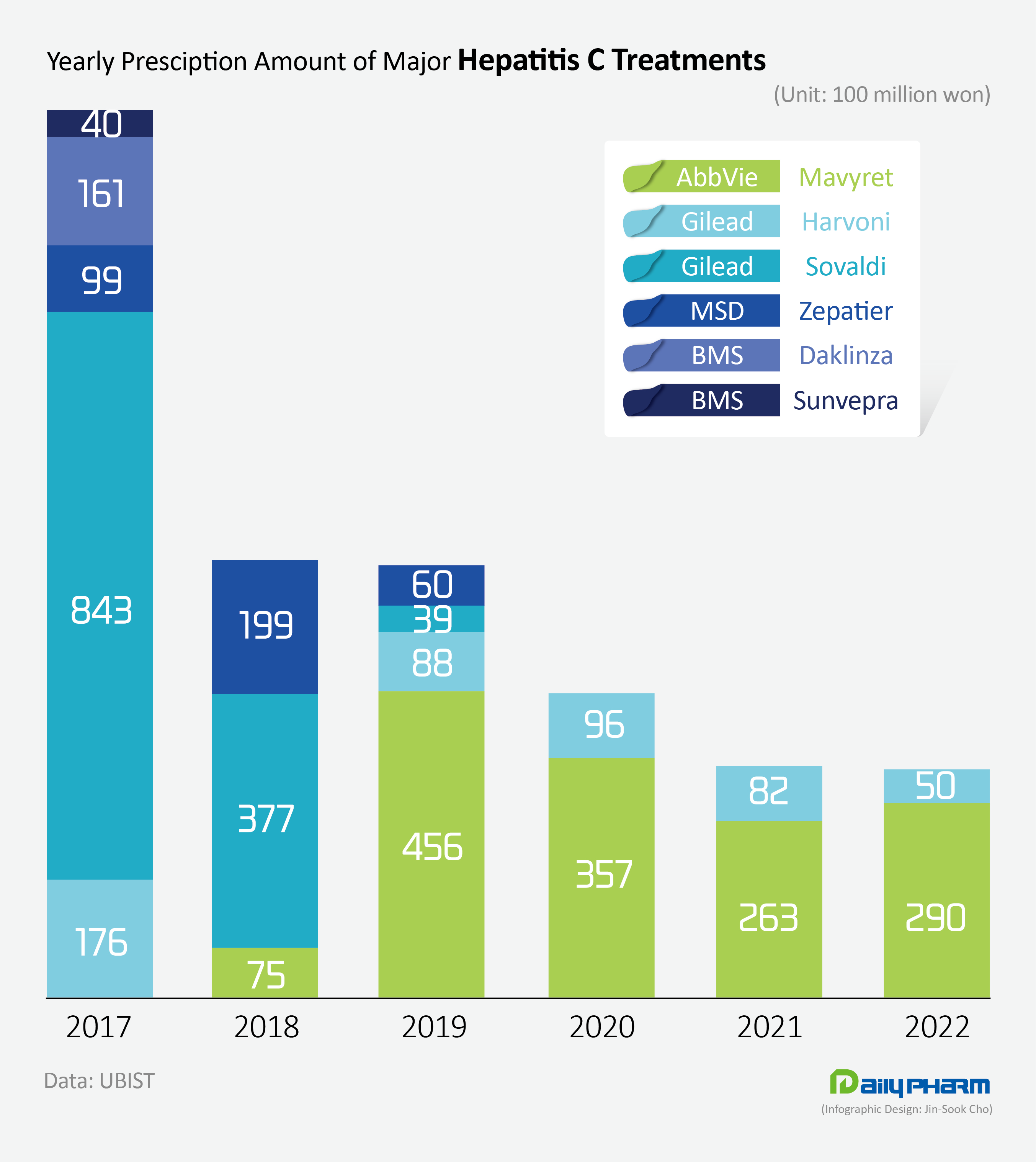
The size of the oral HCV treatment market has steadily decreased from KRW 135.3 billion in 2017 to KRW 73.7 billion in 2018, to KRW 65.1 billion in 2019, to KRW 47.4 billion in 2020, to KRW 35.1 billion in 2021, then to KRW 34.2 billion in 2022.
Compared to 2017, when the market had expanded to its maximum, the market had shrunk to one-fourth its size in 5 years.
◆Increased cure rate had contracted market size...All HCV drugs other than Mavyret·Harvoni earn less than KRW 100 million In the pharmaceutical industry, the cause of market contraction is due to the characteristics held by HCV treatments.
Before the introduction of direct-acting antivirals (DAAs) like Mavyret, HCV had been a very critical condition.
However, the treatment effect of HCV drugs had increased dramatically with the introduction of BMS’s Daklinza and Sunvepra.
Then, Gilead Sciences' Sovaldi and Harvoni.
MSD’s Zepatier and Abbvie’s Mavyret followed, enhancing the treatment effect With the treatment effect high enough to be close to a complete cure, the market size quickly contracted with the number of patients being prescribed the drug increasing within the finite number of patients in the market.
With the rapid contraction of the market, some drugs that once dominated the market decided to withdraw from the domestic market.
In March 2021, BMS voluntarily withdrew the authorization for its for Daklinza and Sunvepra.
In June, Roche also voluntarily withdrew its injectable HCV treatment Pegasys from the domestic market.
The situation is also similar for drugs other than Mavyret.
Prescription performance of drugs that had occupied the market after Daklinza and Sunvepra, such as Zepatier, Sovaldi, and Harvoni are converging to nearly 0.
In the case of Zepatier, its prescription sales had recorded KRW 19.9 billion in 2018, but then fell rapidly to record less than KRW 50 million last year.
Sovaldi’s sales had also fallen to less than KRW 10 million last year from the KRW 84.3 billion in 2017, and Harvoni’s sales had fell to KRW 5 billion from the 40.9 billion in 2016.
◆Gilead releases a new drug for the first time in 5 years...makes winning bid with low price
The variable is the new HCV treatment released by Gilead Science.
Gilead had released Epclusa and Vosevi, its next-generation HCV treatments in November last year.
These were the first new drugs for HCV released by Gilead in 5 years after Sovaldi and Harvoni.
Epclusa is a pan-genotypic treatment like Mavyret.
Although its treatment period is 12 weeks, 1 month longer than Mavyret, it has a more convenient means of administration of one pill once daily compared to three pill once daily administration of Mayvert.
Its price had been set lower than Mavyret.
Epclusa is priced at KRW 117,030 per tablet and Vosevi at KRW 120,836 per tablet.
In terms of total treatment cost, Epclusa costs KRW 9,830,520 and Vosevi 10,150,224.
This is cheaper than the KRW 10,922,352 of Mavyret.
Gilead plans to regain the glory it occupied in the past with treatments and Harvoni with its more competitive price than Mavyret.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









