- LOGIN
- MemberShip
- 2025-12-24 03:48:48
- Keytruda leads the market for 3 consecutive years
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-02-22 05:54:13
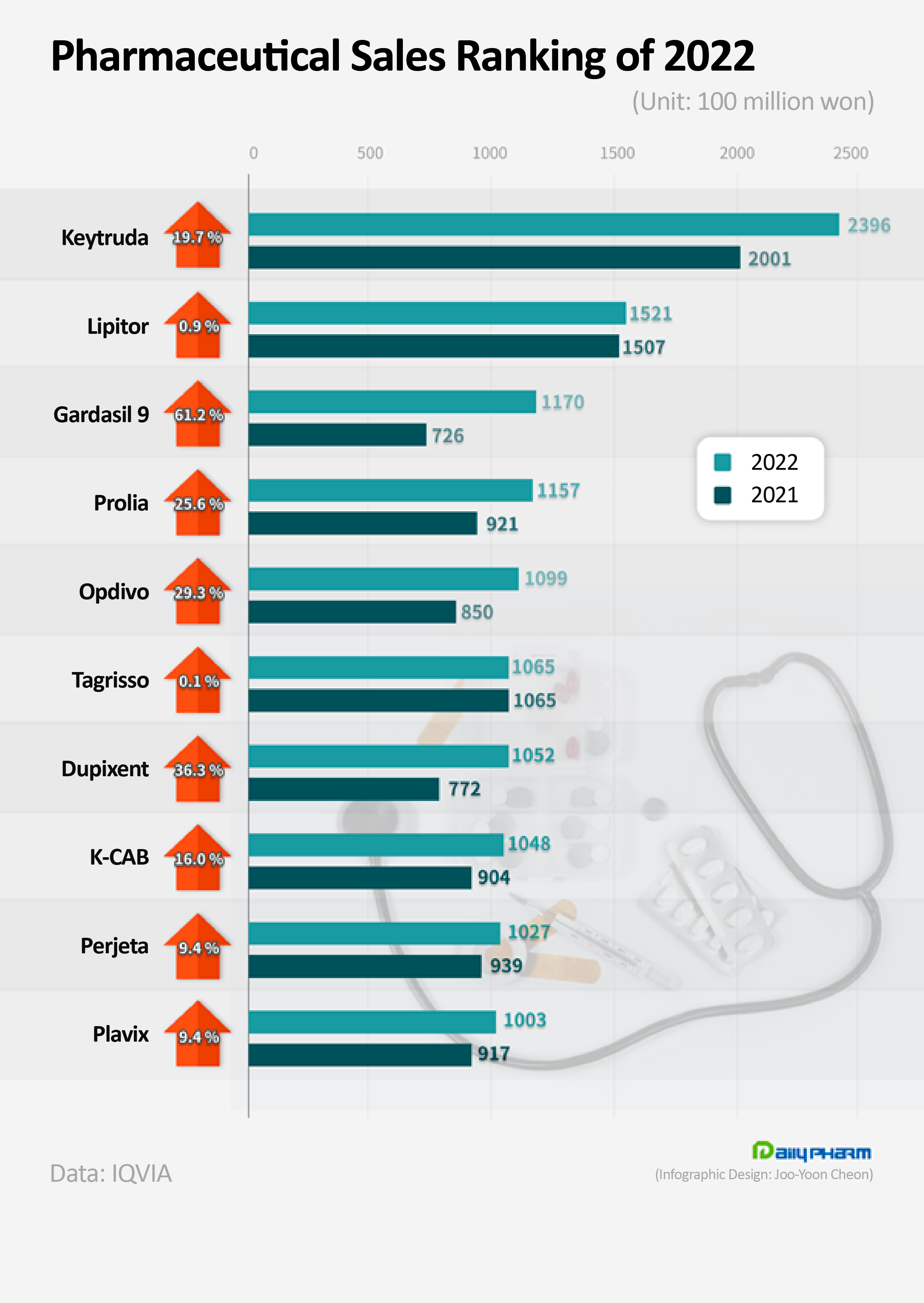
The immuno-oncology drug Keytruda has topped the rank in pharmaceutical sales for 3 consecutive years.
Also, new drugs from multinational pharmaceutical companies, such as Gardasil 9, Prolia, Opdivo, and Dupixent showed strong growth and joined the KRW 100 billion club after exceeding KRW 100 billion in sales last year.
On the 22nd, according to the pharmaceutical research institution IQVIA, MDSD's Keytruda's sales topped the market by recordeingKRW 239.6 billion last year.
This is a 19.7% YoY increase from the previous year.
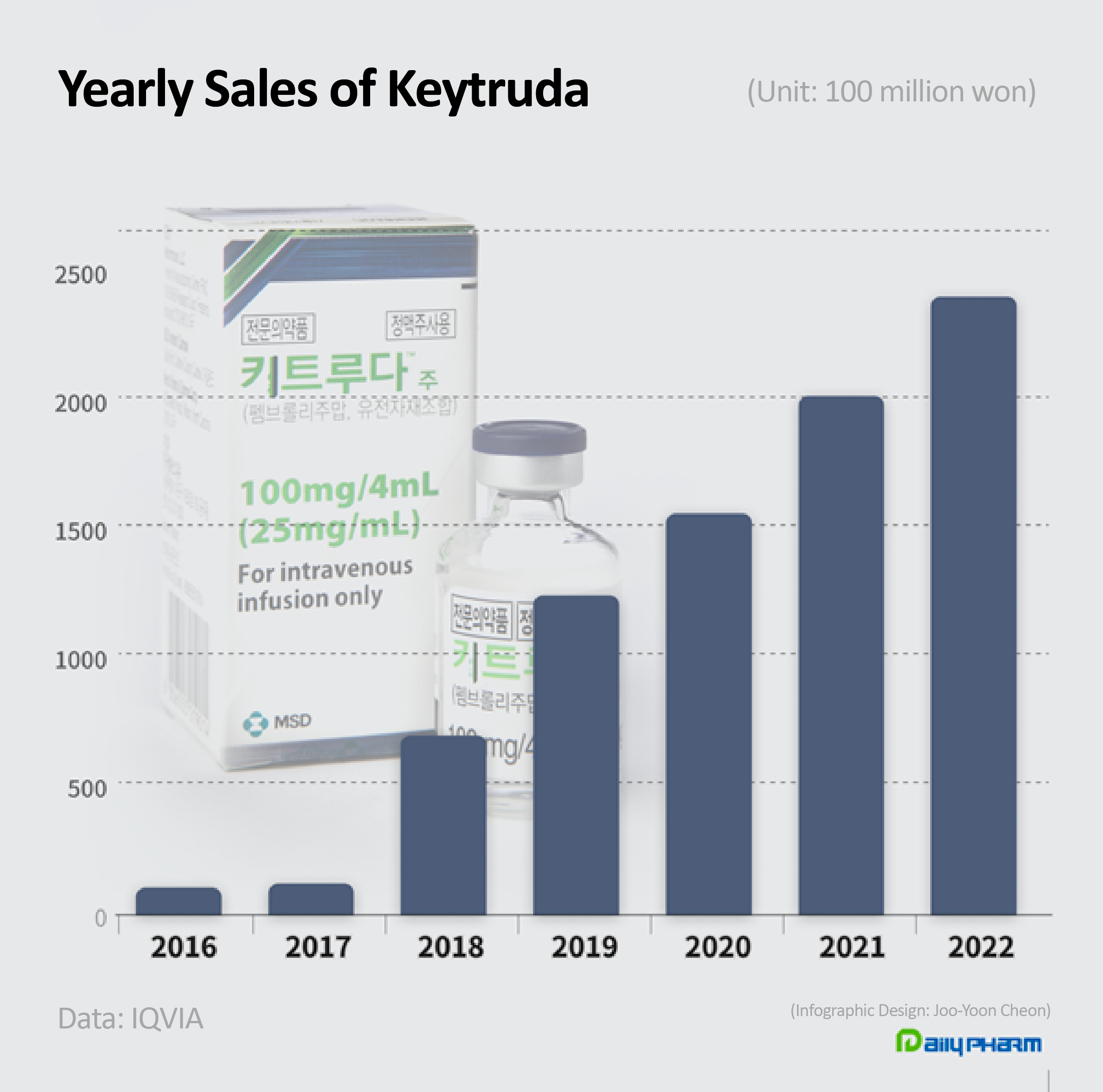
This is the third consecutive year the drug has held the lead after taking the top spot for the first time in 2020 with sales of KRW 155.7 billion.
Also, Keytruda’s sales exceeded 200 billion won for 2 consecutive years since 2021.
The drug is currently approved for 16 cancers: ▲Lung cancer, ▲head, and neck cancer, ▲ Hodgkin lymphoma, ▲urothelial carcinoma (bladder cancer), ▲esophageal cancer, ▲ melanoma, ▲renal cell cancer (kidney cancer), ▲endometrial cancer, ▲stomach cancer, ▲small intestine cancer, ▲ovarian cancer, ▲pancreatic cancer, ▲biliary tract cancer, ▲colorectal cancer ▲triple negative breast cancer, and ▲cervical cancer.
It is indicated for the largest number of cancer types among cancer immunotherapies approved in Korea.
In the early years of its release, in 2016 and 2017, its sales had only been KRW 100 billion and KRW 12.2 billion.
However, its sales started to surge with reimbursement approval.
After reimbursement was applied for non-small-cell lung cancer (NSCLC) in August 2017, its sales soared over fivefold to KRW 70.3 billion in 2018, and then exceeded KRW 100 billion by 2019.
In 2020, the drug outsold the then-lead Lipitor and rose to the lead.
Last year, Keytruda continued strong growth amid favorable and unfavorable events.
Keytruda’s insurance ceiling price had fallen 25.6% with its reimbursement extension to first-line treatment of NSCLC in March this year.
Its sales in Q1 last year had fallen 8.4% compared to the same quarter the previous year due to the price cut – to KRW 40.4 billion – but it recovered its momentum after the benefits from its first line reimbursement were applied in earnest.
When considering the price cut that was applied to Keytruda, its sales volume has increased by over 60%.
MSD’s HPV vaccine Gardasil 9 ranked third last year with sales of KRW 117 billion, rising 61.2% from the previous year.
Gardasil 9 is an improved version of the company’s Gardasil, which offers protection for four serotypes (6, 11, 16, and 18).
Gardasil 9 offers protection for five more serotypes (31, 33, 45, 52, 58) than Gardasil.
Also, It contains the most HPV types among cervical cancer vaccines.
Vaccinations among males for Gardasil 9, which was released at the end of 2016, have also been rising every year with the news spreading that Gardasil 9 offers protection for HPV-related diseases other than cervical cancer, such as anal cancer, genital warts, and precancerous lesions.
Also, the revaccination rate has also risen greatly among adults who already received vaccination after the recommended age was expanded from 9-26 to 27-45 in July 2020.
Sales of Gardasil 9 had increased over twofold in 2 years from KRW 42.5 billion in 2020 to exceed KRW 100 billion for the first time last year.
Amgen’s Prolia ranked fourth raising KRW 115.7 billion YoY last year.
Prolia, a biological osteoporosis treatment that targets the RANKL protein essential for the formation, activation, and survival of osteoclasts that destroy the bone, was released in November 2016 in Korea.
Its sales started to rise after it was applied reimbursement as a second-line treatment in 2017.
After additionally being approved for reimbursement in the first line from April 2019, Prolia’s sales rose explosively and its annual sales exceeded KRW 100 billion for the first time.
Prolia is copromoted by Chong Kun Dang in Korea.
Sales of Ono Pharmaceutical’s cancer immunotherapy Opdivo increased 29.3% YoY to record ₩109.9 billion this year.
Opdivo, which was approved in 2015, recorded a high growth rate of 64.7% in two years from the 66.7 billion it had earned in 2020, and its annual sales exceeded 100 billion for the first time last year.
Sales of Sanofi’s atopic dermatitis treatment Dupixent rose 36.3% YoY to record ₩105.2 billion last year.
Dupixent is the first targeted biologic for the treatment of moderate-to-severe atopic dermatitis that is not well controlled with prescription topical therapies or who cannot use topical therapies.
Sales of Dupixent, which was approved in March 2018, increased rapidly after it was approved for reimbursement for severe atopic dermatitis in January 2020, and exceeded KRW 100 billion last year.
Roche’s Perjeta’s annual sales rose 9.4% YoY to record KRW 102.7 billion last year.
Perjeta is approved for use in combination with docetaxel and trastuzumab in patients with metastatic or unresectable locally advanced HER2-positive breast cancer who have not received anti-HER2 therapy or chemotherapy for metastatic breast cancer.
The drug was approved for reimbursement to treat patients with metastatic or unresectable locally advanced HER2-positive breast cancer who have not received anti-HER2 therapy in 2017.
After the drug was granted selective reimbursement in May 2019, trastuzumab and combination therapy settled as the standard adjuvant therapy and its sales exceeded KRW 100 billion.
Among new drugs developed by domestic pharmaceutical companies, HK.Inno.N’s K-CAB’s sales rose 16.0% YoY to record KRW 104.8 billion and ranked eighth last year.
K-CAB, which was released in March 2019.
It has a new mechanism of action that inhibits gastric acid secretion by competitively binding to the proton pump and potassium ion located in the final stage of acid secretion.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









