- LOGIN
- MemberShip
- 2025-12-24 02:17:39
- 5th time the charm for Tagrisso’s reimbursement extension?
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-03-24 05:48:27
Although other procedures such as deliberation by the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee remain, the fact that it had overcome the highest barrier to reimbursement, the Cancer Disease Deliberation Committee review, 4 years after its indication was expanded to the first line, is regarded an achievement.
HIRA’s CDDC held its second 2023 Reimbursement Standard Deliberation Meeting for Anticancer Drugs on the 22nd and established reimbursement standards for Tagrisso.
The CDDC determined it was appropriate to set reimbursement standards for Tagrisso as a ‘first-line treatment for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) mutations.’ With the decision, the drug was able to pass the first gateway for third-generation EGFR-targeted anti-cancer therapies to receive reimbursement as a first-line treatment.
◆Passes CDDC review after 5 attempts...sees fruition 4 years after indication expansion Tagrisso is a third-generation targeted anticancer therapy that targets the EGFR mutation.
It inhibits both the EGFR mutation and T790M mutation that are represented by L858R and Exon 19 deletions.
As the drug has a high blood-brain barrier (BBB) permeability than first and second-generation EGFR-targeted therapies, Tagrisso has shown a superior effect in patients with brain metastasis.
Tagrisso, which added its first-line indication in December 2018 in Korea, attempted to extend its reimbursement to the indication in 2019.
However, the drug was unable to receive reimbursement as a first-line treatment for over 4 years.
AstraZeneca had attempted reimbursement for Tagrisso at the CDDC level 4 times since 2019 and failed every attempt.

However, the favorable stance faltered with the release of the Asian subgroup analysis results from a global Phase III trial in 2019.
The FLAURA trial assessed the efficacy and safety of Tagrisso in the first line, and the Asian subgroup analysis results of this global trial had risen as a barrier to its reimbursement as its hazard ratio (HR) was 0.995.
An HR of 0.995 indicates that the difference between Tagrisso and the control group is 0.005, which could be interpreted as the difference being insignificantly small.
After such results were disclosed, the CDDC in October 2019 decided to defer its decision until the full data from the Phase 3 FLAURA trial was released.
The company made its second attempt, submitting the overall OS data of FLAURA in 2020.
However, due to the rapid spread of COVID-19, the CDDC meeting that was set for February of the year had been pushed back and canceled several times and finally held at the end of April.
The reimbursement standards for the drug had not been set then either.
Although AstraZeneca expressed their will to accept most of the cost-sharing plan proposed by the government in consideration of the Asian subgroup data, the reimbursement fell through due to strong opposition from committee members that raised the issue of the drug’s clinical efficacy.
In September of the same year, the company made its third attempt powered by results from the FLAURA China study that confirmed improved OS in Asians.
The FLAURA China trial data analyzed a cohort of 136 Chinese patients that included 19 Chinese patients from the global FLAURA trial as well as 117 patients from a trial that had been separately conducted.
Results showed that the median PFS of the Tagrisso group was 17.8 months, which was comparable to the results from the global study.
Median OS in the Tagrisso group was 33.1 months, 7.4 months longer than the 25.7 months in the control group.
This is a higher OS improvement than the 6.8 months identified in the global trial.
The third reimbursement extension discussions for Tagrisso were made in April 2021.
The third attempt also resulted in failure.
At the time, CDDC members concluded that the OS value from the FLAURA China trial lacked statistical significance.
After the third attempt failed, patient groups rose to the occasion.
After Tagrisso's failure to receive reimbursement in April, 1,713 lung cancer patients and their families sent a joint statement to the government imploring the government to extend Tagrisso’s reimbursement to the first line as in many major countries.
Academic societies also criticized how only Tagrisso is not being reimbursed as first-line treatment in Korea.
3 months after its third setback, AstraZeneca applied for the 4th time to extend reimbursement.
This time, the company adopted a strategy of narrowing part of its reimbursement standards.
The company excluded Exon 21 mutation from the 'EGFR exon 19 deletion or exon 21 (L858R) mutation NSCLC’ it had been indicated for.
The company narrowed the criteria to 'first-line treatment for patients with EGFR exon 19 deletion and brain metastases' and reapplied for reimbursement.
The plan was to increase the clinical value of the drug by excluding the patient group that showed a relatively small difference in efficacy from the control group.
However, Tagrisso’s reimbursement extension was rejected at the CDDC meeting that was held in November 2021.
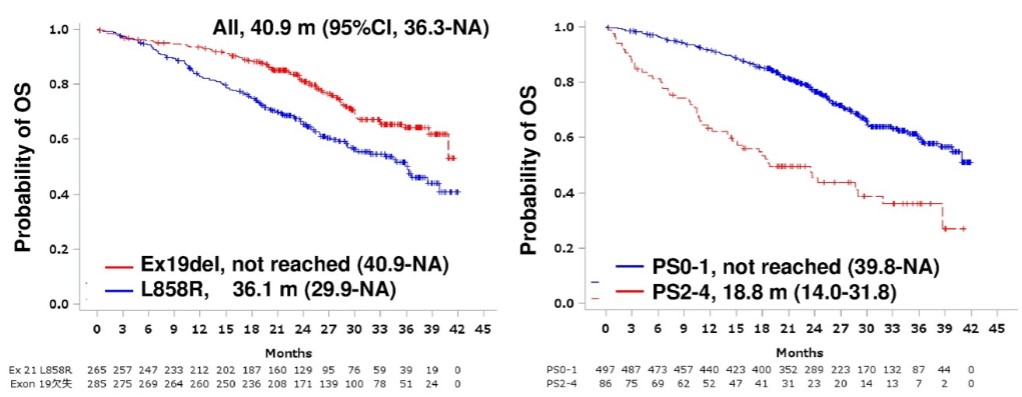
OS results according to gene mutation status (Data: ESMO) This reluctant sentiment on Tagrisso’s reimbursement extension was reversed at the end of last year with the release of large-scale real-world data on Tagrisso’s use in the first line in Asia and Europe.
Analysis of real-world data on 660 Japanese patients confirmed a longer progression-free survival period (20.0 months) and an overall survival period of more than 3 years (40.9 months) than those identified in the Phase III trial.
With this data, the company put an end to Tagrisso’s efficacy controversy in Asia.
AstraZeneca took on its 5th challenge with this new data and a plan to lower drug prices as supplements.
The patient organizations’ petition requestion reimbursement extension also added support.
The 'Petition regarding the request for first-line treatment of the lung cancer treatment Tagrisso’ that was uploaded to the e-People website in February was sent to the National Assembly Health and Welfare Committee for deliberation after receiving over 50,000 consents.
As a result, after 5 attempts in the course of the past 4 years, Tagrisso finally made it through the first barrier to its reimbursement and passed the CDDC review.
◆Can it be reimbursed within the year?
Depends on DREC progress Although it has passed the high barrier of CDDC review, many procedures still remain for its reimbursement extension.
For anticancer drugs, CDDC review is only the first step of many.
The agenda has to pass HIRA’s Drug Reimbursement Evaluation Committee (DREC), and then undergo pricing negotiation with the NHIS, then pass MOHW’s Health Insurance Policy Deliberation Committee (HIPDC) review to complete all the procedures required to extend benefits.
Tagrisso is subject to the risk-sharing agreement (RSA) scheme and must pass the pharmacoeconomic evaluation.
HIRA’s statuary evaluation period is set to 120 days or less, but it is common for HIRA to exceed the set deadline if the company is required to submit supplementary data.
After completing discussions with HIRA and passing DREC review, the company has to conduct drug pricing negotiations with NHIS for up to 60 days.
Within 30 days from the period, MOHW’s HIPCD will deliberate and then issue a notification on the new drug price and then list the drug for extended reimbursement.
In other words, at maximum, Tagrisso’s reimbursement extension is set to be made by the end of this year.
The decisive step in advancing or delaying this timing of Tagrisso's reimbursement is expected to depend on DREC’s stage, where the pharmacoeconomic evaluation takes place.
If DREC requests supplementary data repeatedly, reimbursement listing may be delayed indefinitely.
In fact, several anticancer drugs have not been deliberated for over a year at the DREC level after passing the CDDC review.
In the case of MSD's Keytruda, which succeeded in extending reimbursement to the first-line treatment of NSCLC after 4 years, Keytruda’s reimbursement agenda was presented to DREC 6 months after passing the CDDC review in July 2021.
Although the company showed a high willingness to negotiate the reimbursement of Keytruda, the process was only completed eight months after passing the CDDC review due to a delay in its schedule, such as an unsuccessful submission for the DREC review in November 2021.
AstraZeneca plans to make the best efforts to extend Tagrisso's reimbursement within the year.
The company said, "We welcome the CDDC’s decision and would like to express our thanks to the government and committee members for making efforts to enable this.
We will continue to do our best to complete the procedures that remain and receive the reimbursement decision”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









