- LOGIN
- MemberShip
- 2025-12-24 00:24:16
- Hanmi internationally introduces 7 new drug candidates
- by Hwang, Jin-joon | translator Kim, Jung-Ju | 2023-04-04 05:53:06
(Photo AACR) Hanmi Pharmaceutical is revealing its messenger ribonucleic acid (mRNA) cancer vaccine and new targeted anticancer lead compounds.
The company reinforced its anticancer pipeline by adding next-generation anticancer drug technologies and targets to its existing major anticancer drug pipeline that has been making smooth progress in preclinical trials.
According to industry sources on the 2nd, Hanmi Pharmaceutical will be presenting 7 abstracts related to its anticancer pipeline at the American Association for Cancer Research (AACR) Annual Meeting 2023 held in Orlando, Florida from the 14th (local time) to the 19th.
At AACR 2023, the company will unveil abstracts about 2 lead compounds in its new anticancer pipeline.
One will be about enhancing antitumor activity by inhibiting the YAP/TAZ-TEAD that targets the Hippo pathway, and the other is about an mRNA cancer vaccine.
Also, Hanmi Pharmaceutical will be making abstract presentations on how its novel SOS1 inhibitor, HM99462, demonstrates antitumor activity against KRAS-mutant cancers, how its EZH1/2 dual inhibitor HM97662 demonstrates antitumor activity in T-cell lymphoma, the effect of combining use of HM97662 with an immune checkpoint inhibitor, the antitumor effect of its IL-2analog, HM16390, and how BH3120, a bispecific antibody that targets the G-1BB and PD-L1 simultaneously, stimulates T cells in tumor tissue preferred manner.
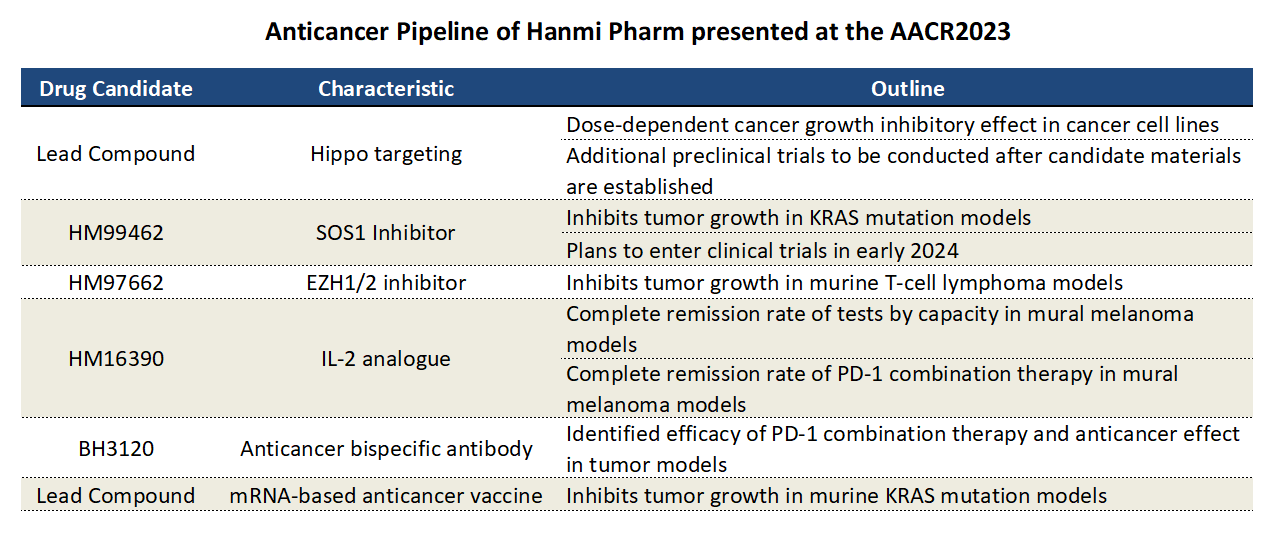
Its safety profile was also confirmed in vitro.
Hanmi’s lead compounds that target the Hippo pathway also showed effective tumor growth inhibition activity within tolerable doses in cancer cell xenograft mouse models.
The company plans to initiate preclinical studies after establishing a candidate substance for a preclinical study.
In addition to the lead compound for the new anticancer drug that targets the Hippo pathway, Hanmi Pharmaceutical also disclosed the results of preclinical studies related to 4 candidates in its major pipeline through abstracts in advance.
According to the abstracts, the SOS1 inhibitor HM99462 showed effective tumor growth inhibition activity within tolerable doses when administered alone in cancer cell xenograft mouse models harboring various KRAS mutations.
Also, the combination of HM99462 with KRAS G12C or MEK inhibitors led to synergistic anti-tumor activity in both in vitro and in vivo models.
HM99462 is currently in IND enabling GLP-toxicity studies and is palnning to initiate a clinical study in early 2024.
At the meeting, Hanmi Pharmaceutical also disclosed the antitumor activity of HM97662 in T-cell lymphoma.
The enhancer of zeste homology 2 (EZH2) and its homolog EZH1 are types of enzymes known to induce solid cancers like blood cancer.
Hanmi confirmed the tumor growth inhibition effect of oral administration of HM97662 in EZH1/2 co-expressed lymphoma cell mouse xenograft model.
The company also presented two papers on preclinical studies conducted on its IL-2 analog, HM16390.
After administering HM16390 once a week for two weeks in mouse models with melanoma, complete response (CR) was observed in 89% and 100% of the mice at 2.1mg/kg and 8.5 mg/kg, respectively.
The median overall survival was also dose-dependently prolonged median overall survival from 15 to 38 days at the dose range of 0.34 to 42 mg/kg.
Also, in mouse models with melanoma, the combined use of HM16390 with PD-1 targeted immune checkpoint inhibitors once a week for 4 weeks, CR was increased from 22% with monotherapy to 88% with combination therapy.
In addition, Hanmi’s Chinese subsidiary, Beijing Hanmi Pharm disclosed preclinical study results of its bispecific antibody that is designed to target two different targets imultaneously with the Pentambody™ platform.
Pentambody is a next-generation, bispecific antibody platform technology that allows one antibody to bind to two different targets simultaneously.
Beijing Hanmi Pharm confirmed that BH3120 showed strong antitumor efficacy in multiple tumor models, and that the combination of BH3120 with a PD-1 antagonist showed synergic effects of continuing to activate the T-cells while reducing immune-related side effects.
In he toxicology studies conducted so far with cynomolgus monkeys, the No Observed Adverse Effect Level (NOAEL) of BH3120 was determined to be 200 mg/kg.
Abstracts of preclinical studies related to mRNA cancer vaccines have not yet been published.
The contents that will soon be disclosed are the preclinical results of a clinical study suppressing tumor growth in a KRAS mutant LL/2 mouse model using a multi-target mRNA-based cancer vaccine.
Previously, Hanmi Pharmaceutical announced that it had successfully developed its own mRNA platform at the JP Morgan Healthcare Conference 2022 held in January last year.
In addition to developing a COVID-19 vaccine, the company has been promoting the application of the mRNA platform in the fields of metabolic diseases, anticancer, cardiovascular and renal diseases, enzyme replacement therapy, etc.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









