- LOGIN
- MemberShip
- 2025-12-24 00:23:30
- Rise of new drugs... subject of NA and public petitions
- by Eo, Yun-Ho | translator Kim, Jung-Ju | 2023-04-07 05:54:56

Such activities, which would not have happened 5 years ago, are actually arising in Korea.
The emergence of government affairs (GA) and patient advocacy (PA) managers are not irrelevant to the rise of such new drug issues and are more fundamentally related to the industry’s entry into an era of high-priced new drugs.
◆Awakening of the patients and rising public interest=" Please doctor, you are our only hope.
Please save me.’ Times have changed.
Patients and their families that used to cling to their doctors and plead for help now independently search papers and find new drugs in the clinical trials database, Clinicaltrial.gov.
If a drug approved in Korea is stuck in the reimbursement process, civil complaint emails and calls pour into the webpages and offices of relevant ministries including the National Health Insurance Service and the Ministry of Health and Welfare.
The same goes for the pharmaceutical companies that supply the new drugs.
Some have described that the complaints have been "hindering work to the point of paralysis."

However, the current generation, which consists of a larger proportion of highly educated people, has ‘administrative power.’ The rising voice has reached the National Assembly.
The NA questioned and criticized the Ministry of Health and Welfare and its affiliated agencies (HIRA, NHIS) in their audit.
Professional terms related to reimbursement, such as the pharmacoeconomic evaluation exemption system, ICER value, and Cancer Disease Review Committee, are also commonly mentioned.
Pressure from the National Assembly and patients, not from pharmaceutical companies, is imposing an incomparable weight on the health authorities.
It will also inevitably affect the reimbursement listing process.
The PA and GA managers at pharmaceutical companies focus on handling issues arising from these situations.
This is why the government often accuses pharmaceutical companies of triggering this phenomenon.
In a way, it is a reasonable doubt.
When sharing the same interest, patients can work as the best weapon for pharmaceutical companies.
Also, the accusations are not 100% false.
Some companies use patient groups to raise public opinion and criticize the government.
In addition, there are companies that drop the introduction of some drugs due to lack of marketability in Korea despite public criticism, and companies that deliberately delay drug price negotiations to be included in the government’s coverage enhancement plan.
They all do exist.
However, patients are now a 'double-edged sword' for the companies as well.
Patients are moving faster than the listing plan set by the companies after approval, and the reimbursement standards the company sets through discussion with the authorities arouse impatience and anger amongst ineligible patients.
The PA and GA meet with the National Assembly and patients to explain, clarify issues, and discuss support plans.
Due to the highly sensitive nature of the work, education is essential to avoid crossing the line.
One thing clear is that the position was created due to the prevailing trend, even more so than for the company’s pursuit of profit.
A PA official at a multinational pharmaceutical company said, "Even support programs for non-reimbursed treatments are also required to abide by the Fair Trade Act.
Although people may suspect that we have alternative purposes, I believe everyone will feel the same way when they meet actual patients and their families.
There are times I clash with the company due to such effect.” The official added, “Among the issues patient organizations always raise, there are many opinions that the government (especially the MOHW) should prepare an official communication channel (with dedicated personnel and departments) for patients and implement a system that regularly and continuously holds an ear out to the patients' voices.
It is now the time for both the government and the pharmaceutical industry to accept the patients’ request." ◆Drug become better and more expensive, system’s scope narrows=Patients that were incurable in the past are now allowed to live their life with the benefit of a drug and even dream of a cure.
Advanced cutting-edge new drugs we encounter now offer surprising efficacy and safety.
In addition to one-shot CAR-T therapies, approvals for new drugs with various mechanisms of action such as targeted anticancer drugs, cancer immunotherapies, and ADCs, as well as new drugs applied to specific targets or all-comers are lining up their applications.
Korea’s reimbursement system has also evolved.
Institutional devices for the introduction of high-priced new drugs, such as the Risk Sharing Agreement (RSA) scheme and pharmacoeconomic evaluation exemption system have been prepared, and many drugs have been listed with reimbursement thanks to such systems.
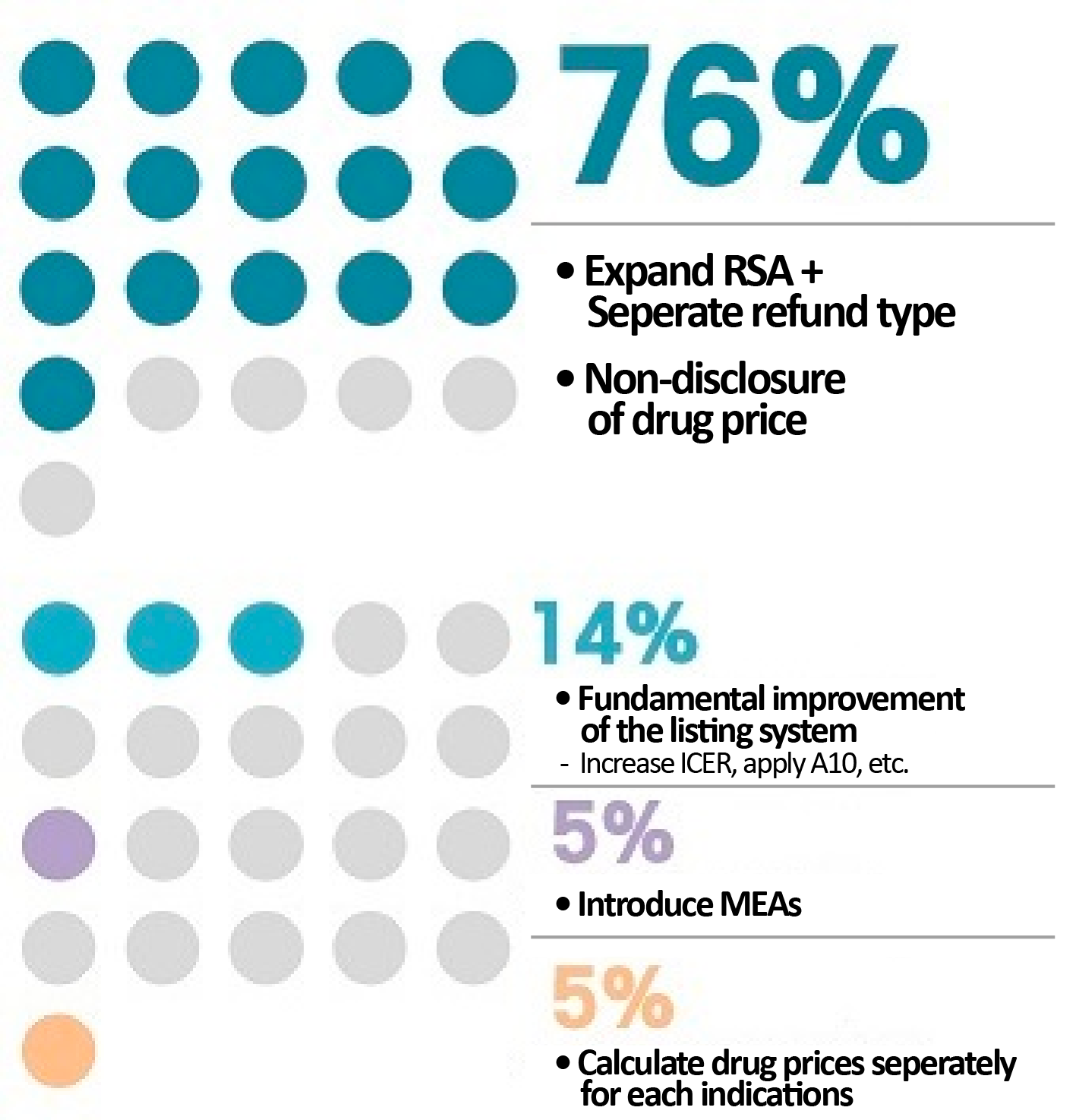
Although the companies applied for reimbursement listing, more and more drugs remain in the discussion stage for 1 to even 3 years at most.
Also, the rejection rate in the CDDC stage, which is an essential gateway to reimbursement for anticancer drugs, has peaked.
A study found that 76% of respondents in the industry pointed to the expansion of the RSA system and the separation of the refund-type RSA as ways to improve the current insurance reimbursement system, and 16% answered that the reimbursement listing system needed fundamental improvement.
In other words, an increasing number of pharmaceutical companies are looking to change the reimbursement system to list their new drugs, rather than finding a way within the system.
GAs play a pivotal role in this context.
If the MA appeals to the MOHW, NHIS, and HIRA on the need for a drug, GAs play a broader role in drawing a public picture.
This is also the reason why an increasing number of GAs in the industry are former NA aides.
However, internal and external conflicts still remain.
Internally, MAs, who are typically pharmaceutical industry experts, have a good understanding of drugs and the drug pricing system, but GAs are often not from the pharmaceutical industry.
In other words, the perception that 'GAs don't know the industry well' exists in compnaies.
One manager explained that internal disputes arise between the MA that speaks on behalf of HIRA, and the GA that speaks on behalf of the NA.
A GA manager from a multinational company said, "I feel most upset when people mistakenly believe that I’m not working because I have to work outside the office to meet various people.
Pharmaceutical companies also follow the trend and communicate better internally.
Only pharmaceutical companies that can create synergy with various internal departments can succeed in leveraging GA.
Integrate the internal message first to progress further."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









