- LOGIN
- MemberShip
- 2025-12-23 22:29:00
- SK Biopharm’s cenobamate secures KRW 700 billion
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-05-12 05:45:00
Cumulative sales of SK Biopharmaceutical’s epilepsy treatment cenobamate exceeded KRW 300 billion in the US.
It had continued to show growth every quarter ever since its release.
Combined with the upfront payment and milestone payments cenobamate has earned more than KRW 700 billion over the past 4 years.
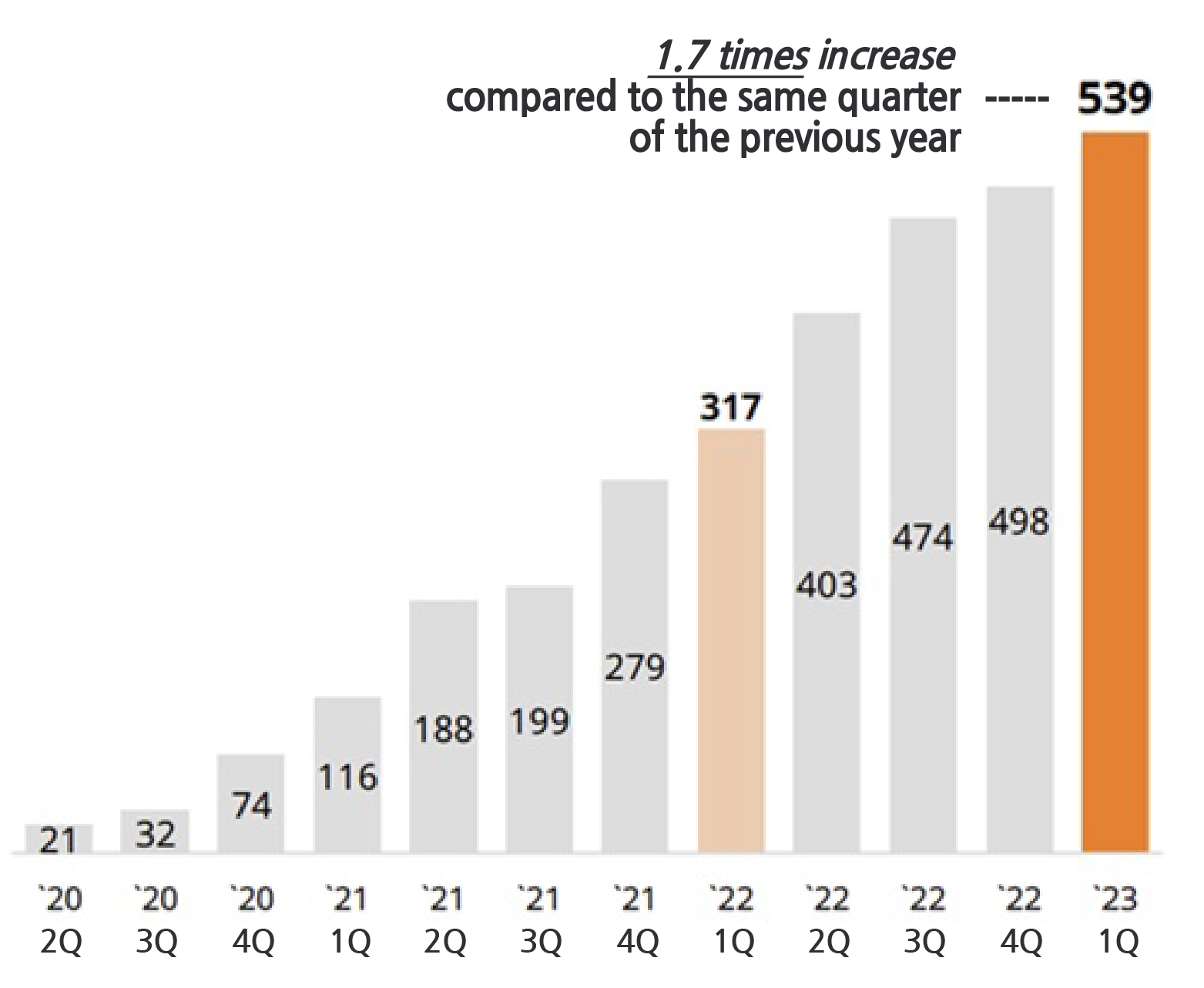
According to SK Biopharm on the 11th, cenobamate posted sales of KRW 53.9 billion in the United States in Q1 last year.
This is a 70.0% increase from the KRW 31.7 billion made in the same period last year.
This also surpassed the previous record of KRW 49.8 billion recorded in the previous quarter by 8.2%, and the sales showed continued growth every quarter since its release.
The total number of prescriptions for cenobamate in the United States is also on the rise.
The total number of prescriptions in the first quarter was about 55,000, up 10% from the previous quarter.
It simultaneously regulates 2 targets related to excitatory/inhibitory signaling that are known to cause epilepsy to reduce seizure frequency.
SK Biopharmaceuticals received approval for cenobamate under the brand name ‘Xcopri’ from the US FDA in November 2019, and has been directly selling the drug through its US subsidiary, SK Life Science since May 2020.
Cenobamate has been growing every quarter since generating initial sales of KRW 2.1 billion in Q2 2020.
In Q1 2021, sales exceeded KRW 10 billion, and quarterly sales exceeded KRW 50 billion this year.
Cumulative sales of cenobamate in the US totaled KRW 314 billion.
Also, Cenobamate has secured over KRW 400 billion as technology fees over the past 4 years.
SK Biopharmaceuticals entered into an exclusive licensing agreement in February 2019 with the Swiss pharmaceutical company Arvelle Therapeutics to transfer technology on cenobamate for up to USD 530 million.
At the time, SK Biopharmaceuticals received an upfront payment of USD 100 million with no obligation of return.
In October 2020, the company entered into an exclusive licensing agreement with Ono Pharmaceutical for Ono to develop and commercialize Xcopri in Japan.
Under the agreement, SK Biopharmaceuticals received an upfront payment of ¥5 billion with no obligation of return, and will also be eligible to receive up to ¥48.1 billion based on the achievement of certain regulatory and commercial milestones, as well as over 10% royalties on net sales generated in Japan.
In November 2021, SK Biopharmaceuticals licensed out 6 new central nervous systems (CNS) drugs including cenobamate to Ignis Therapeutics.
Under the deal, SK Biopharmaceuticals received an upfront payment of USD 20 million, a milestone payment of USD 15 million, and royalties on net sales in the future.
Through the technology export, SK Biopharmaceuticals acquired 150 million shares of Ignis (share amounts to 44.9% including common stock).
And in December 2021, SK Biopharmaceuticals signed a licensing deal with Endo Group for the commercialization of its epilepsy drug cenobamate across Canada.
Under the deal, SK Biopharmaceuticals an upfront payment of USD 20 million.
The company will also be able to receive up to USD 21 million in Canadian dollars based on the achievement of certain regulatory and commercial milestones in the future.
Paladin Labs Inc., a Canada-based operating subsidiary of Endo, will be responsible for all commercial activities related to cenobamate in the region, including its release.
Endo is a global healthcare company headquartered in Ireland.
In July last year, SK Biopharmaceuticals signed a licensing out deal with the Brazilian pharmaceutical company Eurofarma Laboratorios SA for cenobamate.
Under the agreement, SK Biopharmaceuticals will receive an upfront payment of USD 15 million and up to USD 47 million in milestone payments.
Under the licensing out agreement, Eurofarma will be selling cenobamate in 17 Latin American countries including Brazil and Mexico In addition to upfront payments, the company has also received milestone payments upon cenobamate’s approval abroad.
SK Biopharmaceuticals received USD 123.22 million from its European partner Angelini Pharma as milestone payments last year.
Angelini Pharma (formerly Arvelle Therapeutics UK) has collected additional milestone payments after receiving marketing authorization from the European Commission in March last year.
SK Biopharmaceutical’s cash inflow from upfront payments and further milestones from the technology transfer of cenobamate is USD 278.22 million and ¥5 billion.
Based on recent exchange rates, the company had secured about KRW 400 billion through upfront and milestone payments through technology transfer with cenobamate.
Combined with US sales, the drug had brought in over KRW 700 billion.
The company is seeking to expand its sales in the global market.
After its approval in Europe in March 2021, the company released its drug under the product name ‘Ontozry.’ So far, it has been released in 18 European countries, including Germany, England, Italy, Spain, and France.
The company is also speeding up development to expand indications for cenobamate as well as its pipeline.
Cenobamate is undergoing multinational clinical trials to extend its indication to generalized seizures and expand the age group that can be administered from adults to adolescents, and the study has entered Phase III trials in Korea.
An SK Biopharmaceuticals official said, “We plan to conduct aggressive sales activities including improving the incentive system for our sales representatives to encourage sales in the US and by expanding the clientele from epilepsy specialists to general neurologists.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









