- LOGIN
- MemberShip
- 2025-12-24 02:17:14
- JAKi Cibinqo owns strengths in dosage adjustments
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-05-16 05:40:06
Pfizer's JAK inhibitor ‘Cibinqo (abrocitinib)' has embarked on a full-fledged journey to expand its prescriptions.
After landing in major general hospitals at the end of last year, the drug is likely to be registered for reimbursement within the first half of this year.
Cibinqo is a Janus kinase 1 (JAK1) inhibitor approved by the Ministry of Food and Drug Safety in November 2021.
It is the 4th JAK inhibitor introduced to Korea and the second JAK inhibitor drug introduced by Pfizer after Xeljanz.
Unlike Xeljanz, which is only used for ulcerative colitis, Cibinqo is used to treat severe atopic dermatitis.
Treatment options have increased significantly for severe atopic dermatitis starting with the introduction of the injection-type biologic medication and oral JAI inhibitors.
In particular, a total of 3 oral JAK inhibitors are available for patient use in Korea.
Olumiant (baricitinib) and Rinvoq (upadacitinib) have first entered the market and are being used with reimbursement, followed by Cibinqo.
Although Cibinqo is a latecomer, it owns a differentia table property from existing drugs.
Dailypharm met with Jung-Im Na, Professor of Dermatology at Seoul National University Bundang Hospital to hear about its differentiable properties in the field.
Cibinqo comes in three doses: 50, 100, and 200mg.
Although the recommended starting dose is 200mg, the dosage can be adjusted to 100 mg or 50 mg depending on the progress of treatment.
If symptoms worsen with dose reduction, the doctor can increase treatment response again by increasing the dose and using local treatment together (JADE REGIMEN study).
Professor Na said, “JAK inhibitors are fast and effective, but its exit strategy is considered a problem after the patient’s condition improves.
In other words, ending treatment after seeing an effect is difficult with the use of JAK inhibitors.
However still, due to its free dosage adjustments, it is attractive that the dosage can be reduced step by step from 200mg to 50mg.” He added, “No drug can be used for the rest of one’s life.
Therefore, how to complete the treatment well is an important factor, and dose plays an important role.
In particular, JAK inhibitors generally have a short half-life, and therefore disappear quickly from the body, leading to recurrence.
Therefore, you cannot terminate treatment at once,” emphasizing the importance of dosage control.
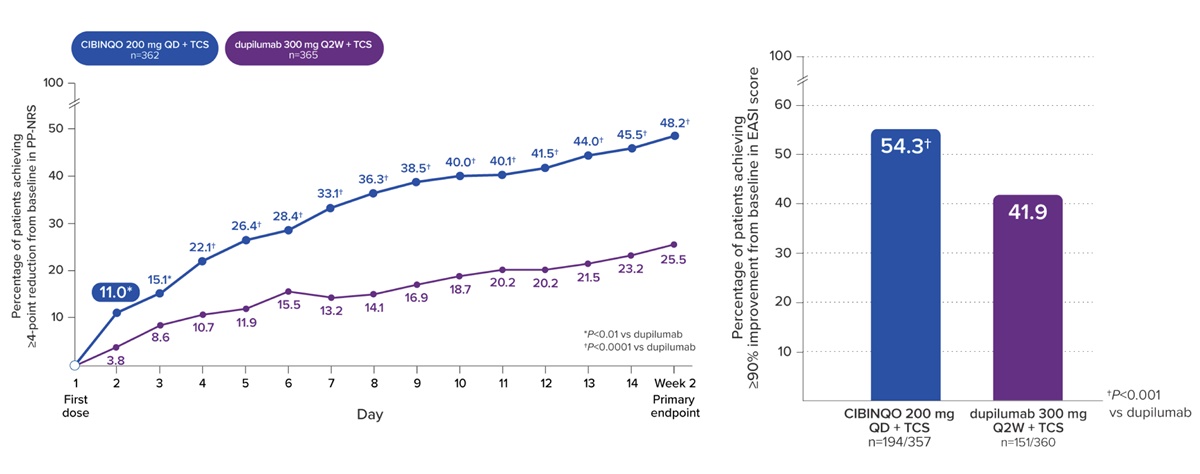
Among Cibinqo’s 7 Phase III clinical trials, the JADE DARE study is a head-to-head clinical trial comparing Cibinqo with Dupixent.
As a result of separately administering Cibinqo 200mg and dupilumab 300mg in combination with a topical treatment for 26 weeks, the proportion of patients who achieved an improvement of 4 points or larger in the Peak Pruritus Numerical Rating Scale (PP-NRS4) at 2 weeks was 48.2% in the Cibinqo group, higher than the 25.5% of the Dupixent group.
The proportion of patients who achieved a 90% improvement in the Eczema Area and Severity Index (EASI-90) at Week 4 was also significantly higher in the Cibinqo group (28.5%) than in the Dupixent group (14.6%).
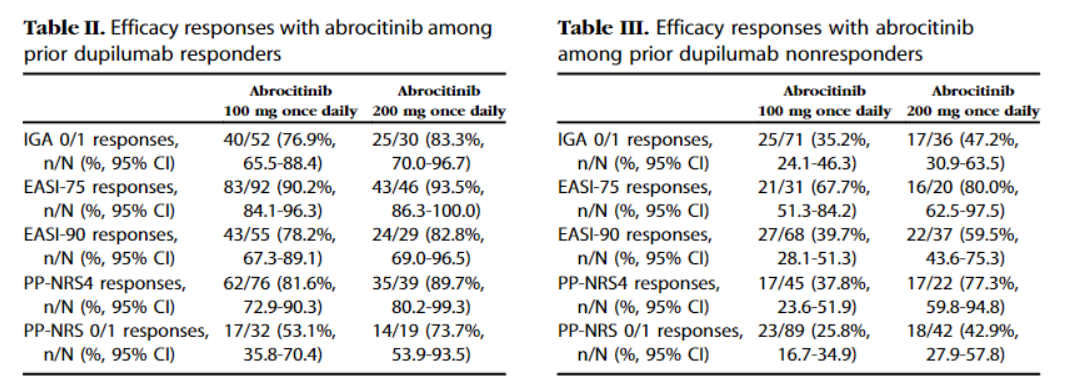
Moreover, the company also conducted the JADE EXTEND study, which studied the effect of switching patients who used Dupixent to Cibinqo.
Patients were divided into those who were responsive to Dupixent and those that were non-responsive to Dupixent.
93.5% and 90.2% of patients who showed a response to Dupixent achieved EASI-75 after switching to Cibinqo 200mg and 100mg, respectively.
PP-NRS4 was 89.7% and 81.6%, respectively.
Among patients who did not respond to Dupxient, the proportion of those that reached EASI-75 was 80% and 67.7%, respectively, and PP-NRS4 was 77.3% and 37.8%, respectively.
Previously, a clinical trial was also conducted with the JAK inhibitor Rinvoq on switching therapy from Dupixent.
However, Cibinqo is the only drug that analyzed the effect of switching administration according to the patient’s presence or absence of an effect with Dupixent.
This is why the trials have raised expectations of JAK inhibitors receiving expanded reimbursement as a replacement therapy when switching from Dupixent.
Professor Na said, “The reason why the JADE EXTEND clinical trial is significant is that there are many patients who do not see an effect while using Dupixent, even though it is a good drug.
However, these patients have to discontinue treatment and use immunotherapies for 3 months and see a deterioration in their condition before switching to a different treatment to receive reimbursement due to limited reimbursement standards.
As Cibinqo has evidence prepared with clinical trials, the trials may be grounds to extend the drug’s reimbursement to switching medications.
Also, For those who have not seen any effect after using Dupixent, the evidence is there to switch to Civinco.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









