- LOGIN
- MemberShip
- 2025-12-23 22:31:18
- Leclaza posts sales of KRW 25 bil in 2 years in Korea
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-05-24 05:32:41
Yuhan Corp’s anticancer drug ‘Leclaza’ is making good sales in the Korean market, and raised sales of KRW 5.1 billion in Q1 alone.
Its efficacy and safety were confirmed in the real world in actual patients at the time of treatment, and the drug is gradually expanding its market influence ahead of its approval as a first-line treatment.
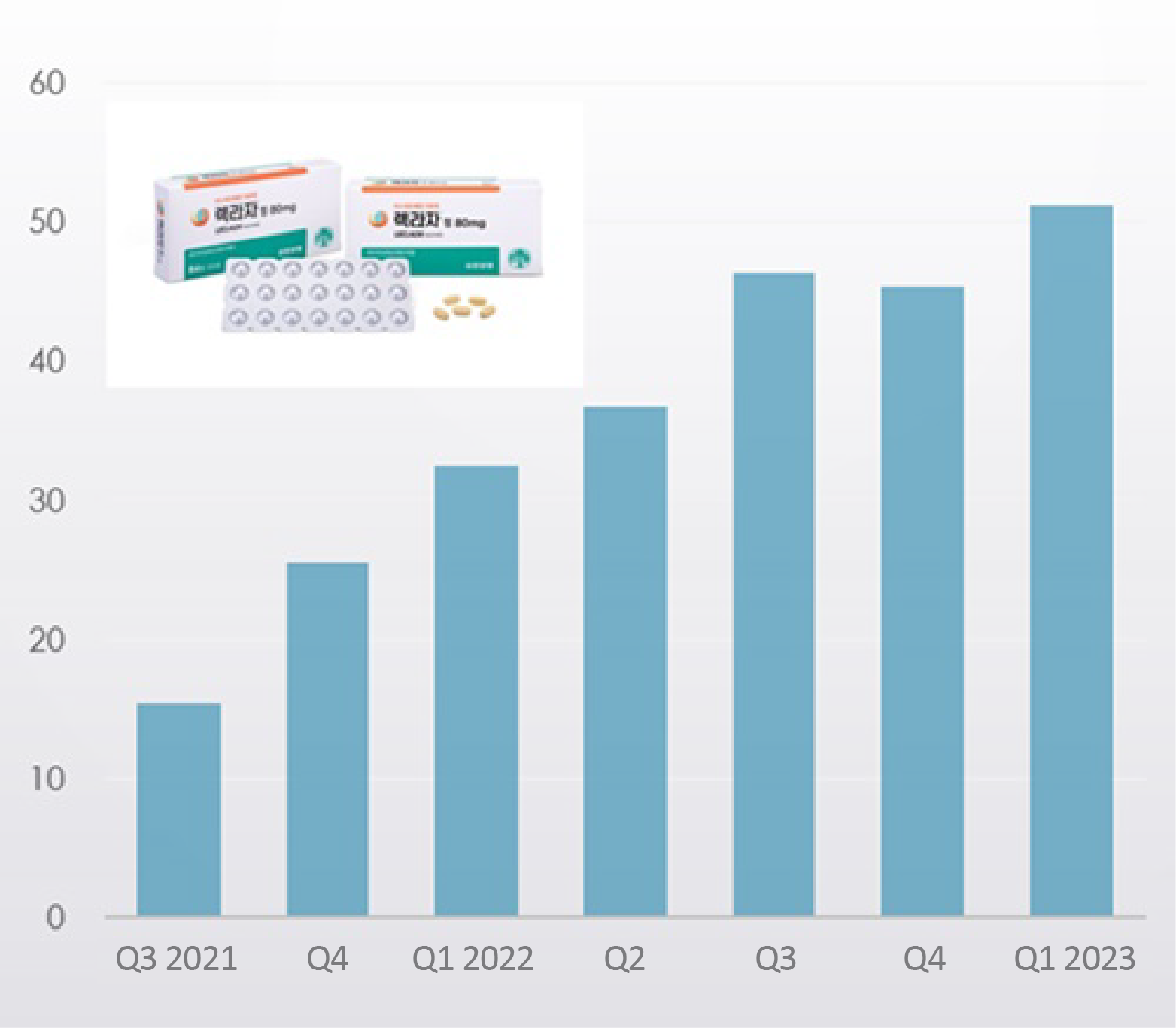
According to the market research institution IQVIA on the 23rd, Leclaza’s sales were KRW 5.1 billion in Q1, up 57.4% YoY.
It is also a 12.9% increase from the KRW 4.5 billion made in the previous quarter.
Leclaza is an NSCLC treatment that was approved as the 31st homegrown novel drug in January last year.
It is a 3rd generation EGFR TKI that inhibits the proliferation and growth of lung cancer cells.
It is currently approved as a treatment for patients with locally advanced or metastatic NSCLC who developed resistance after being previously treated with 1st generation or 2nd generation EGFR-TKIs.
The drug entered the market in earnest after being listed for reimbursement in the National Health Insurance Service in July 2021.
The drug recorded sales of KRW 1.5 billion and KRW 2.6 billion in Q3 and Q4, respectively.
Last year, its quarterly sales had risen to the KRW 4 billion range, and continued growing this year.
Cumulative sales made during the 2 years since the release of Leclaza totaled KRW 25.2 billion.
Other homegrown new anticancer drugs that were approved before Leclaza include Il-Yang Pharmaceuticals’ Supect, Dongwha Pharm’s Milican, Chong Kun Dang’s Camtobell, Sam Sung Pharmaceutical’s Riavax, Hanmi Pharmaceutical’s Olita.
None of the products have exceeded annual sales of KRW 10 billion.
At the current rate, Leclaza may likely exceed annual sales of KRW 20 billion this year.
Leclaza is considered to have made a smooth start in the market.
Anticancer drugs that are usually used in large medical institutions, can only be prescribed after the drug passes each institution’s drug committee, therefore, it takes a considerable amount of time before sales are generated after the initial stage of release.
With the added pressure of having to directly compete with outstanding new drug products from multinational pharmaceutical companies, it is not easy for new anticancer drugs developed in Korea to achieve commercial results.
Leclaza passed the drug committee of major large medical intuitions in Korea and is accelerating its market penetration efforts.
The drug is expected to expand further into the market if it receives approval in the first line.
In March, Yuhan Corp applied for approval of Leclaza as a first-line treatment for patients with locally advanced or metastatic non-small cell lung cancer with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation to the Ministry of Food and Drug Safety.
Leclaza demonstrated its efficacy over existing treatments in a global Phase III trial (LASER 301) that was conducted on 393 locally advanced or metastatic NSCLC patients with EGFR mutations.
The trial results had been presented at the European Society for Medical Oncology Congress that was held last year in Singapore.
The company has also been accumulating evidence of its efficacy and effect in the real world.
Lim Sun Min, Professor of Oncology at Yonsei Cancer Center, and Beung-Chul Ahn, Professor of Oncology at the National Cancer Center, recently published real-world data (RWD) on how Leclaza confirmed its safety and efficacy in practice in the journal, Lung Cancer.
This was the first-ever real-world study results announced since Leclaza’s approval, The research team conducted a retrospective study on 103 patients with EGFR T790M mutation-positive NSCLC patients who developed resistance after being previously treated with EGFR-TKI that received Leclaza from January 2021 to August 2022 at Yonsei Cancer Center and the National Cancer Center.
90 of the 103 patients received Leclaza as a second or third-line treatment.
The patients’ primary efficacy endpoint in the study, median progression-free survival (mPFS), was 13.9 months.
This was consistent with the mPFS of 11.1 months confirmed in LASER201, the study that became the basis of Leclaza’s approval.
The objective response rate (ORR) was 62.1%, slightly higher than the 55.3% observed in the LASER201 study.
In terms of safety, the drug was also well-tolerated, similar to previous studies.
The research team explained, “ The real-world study reaffirmed the consistent effect and efficacy of Leanza as a second-line treatment for EGFR T790M mutation-positive NSCLC patients in practice.” Yuhan Corp has invested a total of KRW 93 billion in the Phase III trial for Leclaza.
According to the Financial Supervisory Service, as of the end of the first quarter, Yuhan Corp reflected KRW 93 billion of Leclaza’s development cost as intangible assets.
In 2019, the Financial Supervisory Service set a standard that only R&D projects that have technical feasibility, including those for new drugs, shall be accepted as accounting assets.
The FSS suggested that R&D costs can be turned into assets after initiating Phase III trials for new drugs and receiving approval for its Phase I trial for biosimilars.
As for generic drugs, they can be capitalized after their bioequivalence test plan is approved.
Under such standards, Leclaza’s development costs of KRW 32.6 billion were first recognized as intangible assets in Q4 2020.
Its development costs were reflected as intangible assets after its Phase III trial began in earnest.
Leclaza’s development cost intangible asset increased to KRW 61.4 billion by the end of 2021, and then rose to KRW 88 billion last year, with the added KRW 26.6 billion last year.
In Q1 this year, an additional KRW 5 billion was invested as clinical expense.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









