- LOGIN
- MemberShip
- 2025-12-23 22:31:18
- Sales of Keytruda rose 117%
- by Chon, Seung-Hyun | translator Choi HeeYoung | 2023-05-30 18:59:59
Immuno-oncology drug Keytruda continued its high-altitude march in the domestic pharmaceutical market.
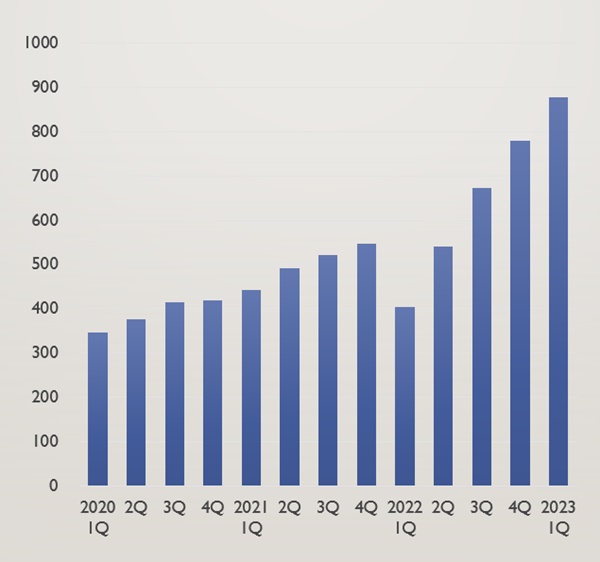
Last year, Health Insurance 2 sales more than doubled as the number of recipients of the leading water-based benefits expanded for 13 consecutive quarters since the first quarter of 2020.
Keytruda further strengthened its solo system by more than doubling the gap with second place.
According to IQVIA, a drug research agency on the 22nd, Keytruda took the lead with sales of 87.8 billion won in the first quarter.
Sales increased by 117.1% in one year from 40.4 billion won in the first quarter of last year.
Released in Korea in 2015, Keytruda is an immune checkpoint inhibitor that inhibits the PD-1 protein on the surface of T-cells of immune cells to prevent binding to the PD-L1 receptor and activates immune cells to treat cancer.
In Korea, 24 indications were approved for 16 cancer types, including melanoma, lung cancer, and head and neck cancer.
Currently, the cancers for which Keytruda can be used are ▲lung cancer ▲head and neck cancer ▲Hodgkin's lymphoma ▲urothelial cancer (bladder cancer) ▲esophageal cancer ▲melanoma ▲renal cell cancer (kidney cancer) ▲endometrial cancer ▲stomach cancer ▲small intestine cancer ▲ovarian cancer ▲ Pancreatic cancer, biliary tract cancer, colorectal cancer (colorectal cancer), triple-negative breast cancer, and cervical cancer reached 16 cases.
It can be used in the largest number of cancer types among immuno-anticancer drugs approved in Korea.
Keytruda showed high growth last year thanks to the favorable news of salary expansion.
In March of last year, the scope of health insurance coverage for Keytruda was expanded as the first treatment for non-small cell lung cancer.
As Keytruda's first-line treatment benefit application effect began in earnest, the growth rate accelerated.
Sales increased by 33.4% from 40.4 billion won in the first quarter of last year to 53.9 billion won in the second quarter.
In the third and fourth quarters of last year, sales were 67.2 billion won and 78 billion won, respectively, raising more than 10 billion won in sales each quarter.
Considering Keytruda's drug price cut, the increase in prescriptions is analyzed to be even greater.
In March of last year, Keytruda's insurance cap was lowered by 25.6% as the benefits range expanded.
In the aftermath of drug price cuts, sales in the first quarter of last year decreased by 26.0% compared to the previous quarter.
However, despite the drug price cut, and the upward trends, Keytrua increased since the second quarter of last year.
It is calculated that the amount used has more than doubled in one year since the first treatment benefit was applied.
It has maintained its lead position for 13 consecutive quarters since taking first place overall in the first quarter of 2020.
The gap with 2nd place Lipitor widened more than twice, establishing a solid solo system.
Amgen's Prolia recorded sales of 35.5 billion won in the first quarter, up 41.6% from the same period last year, ranking third overall.
Released in Korea in November 2016, Prolia is a biopharmaceutical osteoporosis treatment that targets RANKL, an essential protein for the formation, activation, and survival of osteoclasts that destroy the bone.
Since 2017, Prolia began to enjoy an upward trend in sales after benefits were applied only to secondary treatment.
From April 2019, sales of Prolia soared even more as insurance benefits were recognized for primary treatment.
Prolia surpassed 100 billion won in annual sales last year, six years after entering the country.
Prolia is jointly sold by Chong Kun Dang.
Ono Pharmaceutical's immuno-oncology drug Opdivo recorded sales of KRW 33.9 billion in the first quarter, up 35.4% from the previous year.
Opdivo, which was licensed in 2015, stayed in the 10 billion won range until the second quarter of 2021.
It exceeded 20 billion won in the third quarter of 2021 and exceeded 30 billion won in the fourth quarter of last year.
Opdivo recorded a high growth rate of 64.7% in two years from 66.7 billion won in 2020, and stepped on the 100 billion won mark in annual sales for the first time last year.
Sanofi's atopic dermatitis treatment Dupixent recorded sales of 30.9 billion won in the first quarter, up 29.4% from the previous year.
Dupixent is the first targeted biological agent developed for the treatment of moderate to severe atopic dermatitis where topical treatments are not recommended or symptoms are not adequately controlled.
.Dupixent, which received domestic approval in March 2018, received coverage for severe atopic dermatitis from January 2020, and sales expanded rapidly.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









