- LOGIN
- MemberShip
- 2025-12-23 20:49:13
- The only non-reimbursed Evrysdi struggle in the market
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-06-01 05:39:25

However, the company is having trouble posting sales in Korea.
This is in stark contrast to how the same product exceeded the sales of 'Zolgensma' and is comparable to the sales of 'Spinraza.’ The fact that the reimbursement application was not even discussed for nearly 2 years and its reimbursement was blocked acted as a barrier in Korea.
However, the Health Insurance Review and Assessment Service plan to review Evrysdi at its Drug Reimbursement Evaluation Committee (DREC) soon.
According to the market research institution IQVIA on the 31st, the SMA treatment market in Korea had reached KRW 20 billion in Q1, up 15% YoY.
Three SMA treatments are currently available in Korea: ▲Biogen’s ‘Spinraza (nusinersen)’ ▲Roche’s ‘Evrysdi (risdiplam,’ and ▲Novartis’s ‘Zolgensma (onasemnogene abeparvovec).
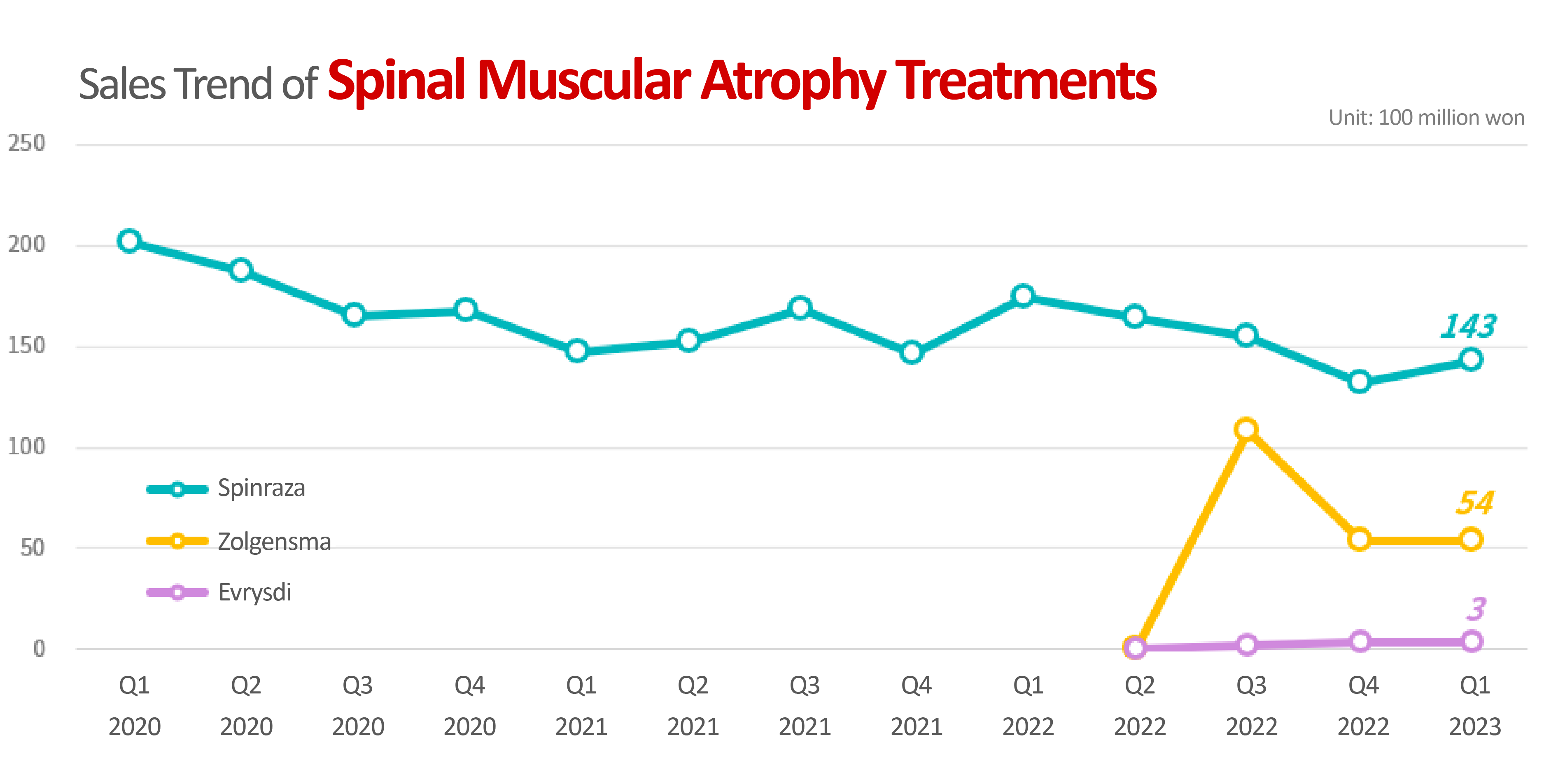
When it was the only drug available in the market, it once exceeded KRW 20 billion in quarterly sales, but it recently showed a decline in sales due to the emergence of its competitors.
In Q1 this year, its sales fell 18% YoY.
Zolgensma, the “one-shot treatment” that only needs to be administered once in a lifetime, came in second with KRW 5.4 billion.
On the other hand, Evrysdi, the only oral drug, recorded only KRW 300 million in quarterly sales.
This is in stark contrast with the global market trend.
In the global market, Evrysdi’s sales exceeded Zolgensma's and are even threatening Spinraza’s sales.
In its Q1 earnings report, Roche announced that Evrysdi's global sales recorded CHF 363 million (approximately KRW 530 billion).
During the same period, Zolgensma posted sales of USD 309 million (approximately 409 billion).
Evrysdi’s global sales followed up to the bottom of Spinraza’s sales, which posted USD 443 million (approximately KRW 586 billion).
SMA is a rare condition in which the SMN1 gene is innately deficient or mutated to result in progressive muscle atrophy.
Globally, SMA occurs in about 1 in 10,000 newborns, and in Korea, it is known that about 30 patients (based on 300,000 newborns) occur each year.
The severity of the condition is closely related to the number of the “backup” SMN2 genes.
The SMN2 gene can produce up to 10% of the SMN protein that SMN1 cannot produce.
In the case of SMA Type 1, the most common and severe form of SMA, if left untreated, over 95% of the motor neurons are damaged within 6 months, and 90% die before the age of 2.
Although the condition is rare, the number of treatment options had increased to three at once with the recent development of new drugs.
Starting with Spinraza in December 2017, Evrysdi in November 2020, and Zolgensma in May 2021 each drug received approval from the Ministry of Food and Drug Safety.
The 3 drugs have different characteristics.
Spinraza has the strength of being the first treatment.
Evrysdi is an oral drug that is easy to administer and relatively inexpensive.
Zolgensma is the most expensive drug in Korea, but it is a gene therapy that can fundamentally treat the disease with one single injection.
The market prospects are more favorable to Evrysdi.
U.S.
analysts have predicted that Evrysdi would post the highest sales in 2026.
They expect that the more affordable oral drug strategy will work for the benefit.
Evrysdi, which has risen to the forefront in major countries such as the United States and Japan, is struggling only in Korea.
Roche released the drug into the domestic market and applied for its reimbursement about a year after approval.
Its sales were recorded only since Q3 2022, 1 year after its launch.
However, unlike the other two drugs that are approved for reimbursement, the non-reimbursed Evrysdi posted less than KRW 500 million in quarterly sales.
Roche applied for the reimbursement of Evrysdi to HIRA in mid-2021, but HIRA delayed discussing Evrysdi’s reimbursement for nearly two years.
The biggest reason was the extension of Spinraza's reimbursement standards.
As the two drugs target the same disease, the authorities planned to discuss the reimbursement of Evrysdi after revising the reimbursement standards for Spinraza, but the discussion on Spinraza took longer than expected, not allowing Evrysdi to even be submitted for review by DREC.
With discussion making way recently, Spinraza and Evrysdi’s reimbursement applications recently passed Drug Reimbursement Standard Subcommittee.
The 2 drugs are scheduled to be presented for review to DREC for the meeting on the 1st of next month.
Expectations are high that the agendas will easily pass the DREC review as they have been discussed for a long time.
As the price is cheaper than its comparators, the drug pricing negotiations are also expected to be completed without difficulty.
If reimbursed, Evrysdi’s sales are expected to expand significantly.
However, since the reimbursement standards are also being extended for Spinraza, it will be difficult for the latecomer Eversdi to compete in the Korean market.
Whether or not the reimbursement for switching will be recognized is also expected to be a variable that determines the extent of Evrysdi’s sales growth.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









