- LOGIN
- MemberShip
- 2025-12-23 20:54:14
- 'Saxenda' continues to dominate weight-loss market
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-06-07 05:38:18

In the first quarter, it recorded sales of KRW 15.9 billion, 53% increase from the previous year, more than doubling the gap with the runner-up product, 'Qsymia (Phentermine+Topiramate).' However, it remains to be seen how much longer Saxenda's dominance will last, as release of promising products such as 'Wegovy (Semaglutide)' and 'Mounjaro (Tirzepatide)' are imminent in the obesity treatment market.
The pharmaceutical industry predicts that the two products which have proven their marketability in the global market will be released in Korea as early as this year.
Saxenda grows 53% in 1 year...
more than doubles the gap with Qsymia According to IQVIA, a pharmaceutical market research institute, on the 5th, Novo Nordisk's Saxenda recorded sales of KRW 15.9 billion in the first quarter.
This is a 53% increase in 1 year, compared to KRW 10.4 billion in the first quarter of 2022.
Saxenda is the world's first obesity treatment approved as a glucagon-like peptide-1 (GLP-1) analog.
It has the same ingredients as the type-2 diabetes treatment 'Victoza,' but with different usage and dosage.
Saxenda has grown rapidly since its domestic release in the second quarter of 2018.
In 2019, the second year after its release, Saxenda dominated the obesity treatment market with sales of KRW 42.6 billion.
Saxenda, unlike existing obesity treatments, is not a psychotropic drug, and therefore gained explosive popularity because it is relatively safe and can be taken over a long period.
The sales of Saxenda have somewhat slowed down due to the impact of COVID-19 in 2020 and 2021.
However, as outdoor activities have become revitalized again from last year, demand for obesity treatments regained its place, and Saxenda's sales soared up to KRW 58.9 billion.
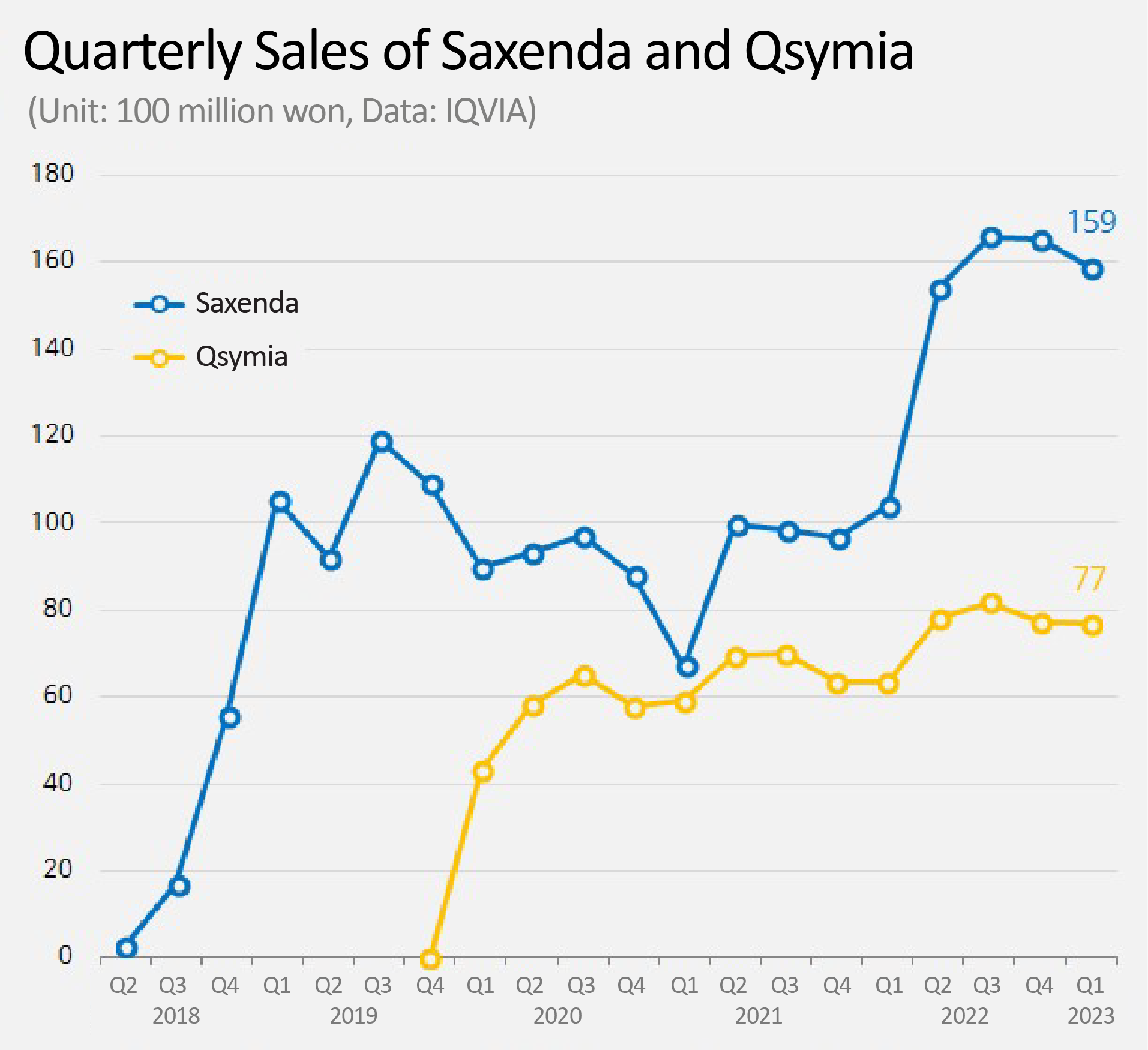
Compared to KRW 6.3 billion in the first quarter of last year, sales has increased by 21%.
It is analyzed that Qsymia was also affected by the recovery of the obesity treatment market.
Qsymia is a combination drug of 'Phentermine' and 'Topiramate,' which Alvogen Korea secured domestic sales right from the US pharmaceutical company Vivus in 2017.
Alvogen Korea started domestic sales with Chong Kun Dang at the end of 2019.
In addition to the advantage that the content of psychotropic ingredient is relatively low even though it is an oral drug, Chong Kun Dang's sales power generated synergy and quickly penetrated the market despite being a latecomer.
However, the gap with Saxenda has widened.
Although Qsymia chased Saxenda's sales up to about 90% with KRW 5.9 billion in the first quarter of 2021, the gap is widening again as the obesity treatment market is recovering.
In the first quarter of this year, the gap between to two products widened by 2.4 times.
Some in the pharmaceutical industry predicts that the gap between the two products will widen further in the future.
This is because obesity treatments containing Phentermine and Phendimetrazine, including Qsymia, were included in the 'list of narcotics and drugs of concern for misuse and abuse' as the non-face-to-face treatment pilot project was implemented.
The government urged caution in prescibing psychotropic drugs such as Qsymia through non-face-to-face treatment.
Saxenda has not been included in the list.
In addition, treatments such as Daewoong Pharmaceutical's Dietamin, Korea Prime Pharm's Phendimen, Huons's Hutermin, etc.
recorded sales of more than KRW 1 billion in the first quarter.
In case of Phendimen, its sales, which recorded just KRW 300 million in the first quarter of last year, increased about 6 times to KRW 1.8 billion in one year, showing remarkable growth.
Wegovy and Mounjaro's impending release...
Saxenda's domination coming to an end It remains to be seen how much longer Saxenda's dominance will last.
Two mega-sized products that have proven their competitiveness in the global market are waiting to be released.
In the pharmaceutical industry, the prevailing view is that Saxenda's dominance will come to an end with the advent of next-generation products such as Wegovy and Mounjaro.

Wegovy is a GLP-1 analog, just like Saxenda.
Novo Nordisk improved its Saxenda, which was administered daily, to weekly administration.
In the US market, in which Wegovy was released earlier, demand for the product soared, with shortages occurring.
In particular, due to its popularity, shortage of Ozempic, a diabetes treatment with the same ingredients and usage, has also occurred.
Even now, there is still a lack of supply of Wegovy in the US.
Due to the circumstances, the official release of Wegovy is being delayed in Korea even after product approval.
The pharmaceutical industry predicts that domestic supply will be possible at the end of this year or early next year.
The domestic release of Eli Lilly's Mounjaro, which is considered a strong competitor of Wegovy, is also imminent.
The Ministry of Food and Drug Safety recently completed a safety and efficacy review on Mounjaro.
Completing the review means that the product approval process will soon begin.
Mounjaro is a GLP-1 analog, just like Saxenda and Wegovy.
After obtaining approval as a type-2 diabetes treatment, Lilly is trying to expand its indications for obesity.
In the case of the Mounjaro, in addition to the mechanism acting on the GLP-1 analog, its mechanism also acts on the glucose-dependent insulinotropic polypeptide (GIP).
Due to this, Mounjaro's weight loss effect was better than that of Wegovy in each drugs' clinical trials.
Lilly also entered a phase 3 clinical trial comparing the effects of Mounjaro and Wegovy on a one-to-one basis.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









