- LOGIN
- MemberShip
- 2025-12-23 20:54:14
- Yuhan presents 4 studies at ASCO...builds reliability
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-06-08 05:33:39

At the ‘2023 ASCO Annual Meeting (ASCO 2023)’ that was held on the 2nd (local time), Yuhan Corp made 4 poster presentations on studies related to its Leclaza, including one long-term follow-up study.
With the results of Janssen’s main MARIPOSA study expected to be released in the second half of this year, initial research results that give a glimpse of the MARIPOSA study have also been updated.
Leclaza is a treatment for non-small-cell lung cancer that was approved as the 31st homegrown novel drug in January 2021.
It is a 3rd generation EGFR TKI that inhibits the proliferation and growth of lung cancer cells.
It is currently approved as a treatment for patients with locally advanced or metastatic NSCLC who developed resistance after being previously treated with 1st generation or 2nd generation EGFR-TKIs.
The drug had raised KRW 25 billion in sales in only 2 years of release.
The company is also awaiting to add a first-line indication to the drug.
Supported by such domestic growth, the company seeks to further its presence with Leclaza in the global market.
Janssen, which introduced Leclaza’s technology, is developing it as a combination therapy with Rybrevant, its EGFR bispecific antibody treatment.
The results of the MARIPOSA Phase III study, which looks at the drug’s effect of combination therapy in the first line, are expected to be announced soon.
Research data that provide a glimpse into the brain metastasis effect of Leclaza and the efficacy of Leclaza as a combination therapy were presented at this year’s ASCO.
First, the long-term follow-up results of the Phase I CHRYSALIS trial, which used EGFR exon 20 mutation treatment Rybrevant with Leclaza in EGFR-mutated NSCLC, were presented as a poster.
The CHRYSALIS trial was the first study to evaluate the efficacy of Rybrevant + Leclaza as a first-line treatment.
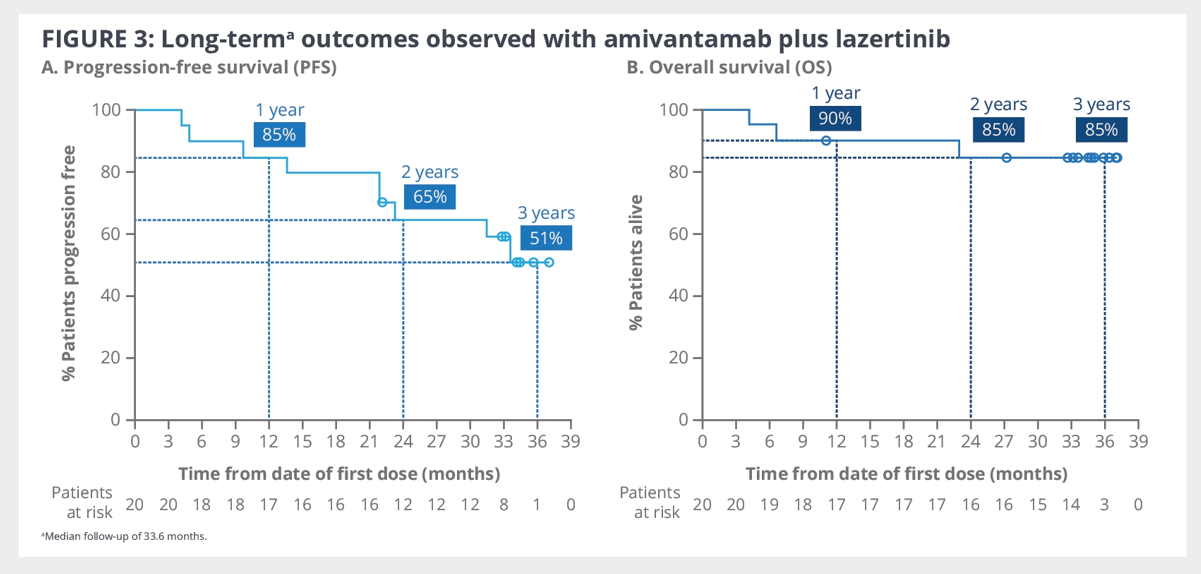
At that time point, key indicators such as overall survival, progression-free survival, and duration of response had not yet reached median values.
Following the poster presentation, an oral presentation on the Cohort D data of the CHRYSALIS-2 study, which evaluated the safety of Leclaza + Rybrevant therapy or Leclaza monotherapy after treatment with Tagrisso, was made at the meeting.
The presented data were the results of the additional analysis conducted on biomarkers from the data that had been announced in 2021.
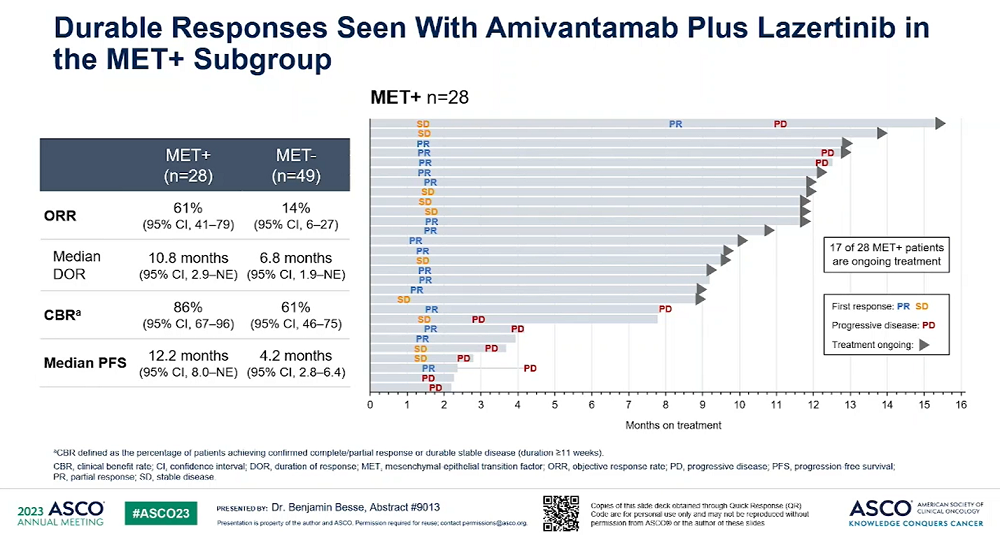
The objective response rate (ORR) of the combination therapy in patients with MET mutations and amplification (28 patients) was 61%, which was higher than 14% in the MET-negative patient group (49 patients).
The median duration of response and median progression-free survival were 10.8 months and 12.2 months in the MET-positive group, respectively, compared to 6.8 months and 4.2 months in the MET-negative patient group.
A domestic phase 2 study that measured the effect of Leclaza in patients with brain metastasis after failure with existing first- and second-generation targeted anticancer drugs in EGFR-positive non-small cell lung cancer was also presented as a poster.
40 EGFR-positive patients with brain metastases after using first- and second-generation treatments were enrolled in the study to evaluate the intracranial activity of Leclaza in patients with asymptomatic or mild brain metastasis.
Patients who failed with conventional treatment were divided according to the presence or absence of the T790M mutation.
The primary evaluation index was intracranial objective response rate (iORR), and the secondary evaluation index was intracranial progression-free survival (iPFS).
The intracranial objective response rate of 38 evaluable patients was 55.3%.
3 patients showed a complete response and 18 patients showed a partial response.
There were only 5 T790M-positive patients, but 4 showed partial response, recording an intracranial response rate of 80%.
Of the 33 T790M-negative patients, 3 showed complete responses and 14 partial responses, recording an objective response rate of 51.5%.
The median overall progression-free survival and the progression-free survival in the T790M positive and negative groups were 15.2 months, 9.9 months, and 15.4 months, respectively.
Intracranial progression-free survival was similar for T790M positive and negative patients, 15.2 months and 15.8 months, respectively.
The research team said, “Leclaza showed significant intracranial activity regardless of the presence or absence of T790M mutation, and satisfied the primary evaluation index with an intracranial objective response rate of 55.3%." This indicates that patients that showed brain metastasis after treatment with targeted therapies may use lazertinib instead of topical treatment." In addition to this, studies that studied predictable biomarkers for the use of Rybrevant + Leclaza after Tagrisso were also announced at ASCO 2023.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









