- LOGIN
- MemberShip
- 2025-12-23 22:31:17
- Will a new treatment option be introduced for gastric cancer
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-06-14 05:38:05

For the past decade, chemotherapy has been the standard first-line treatment for patients with HER2 gene-negative metastatic gastric cancer.
This means that there were no suitable new drugs other than HER2-targeting anticancer drugs available for its treatment.
However, changes have recently occurred in the treatment of gastric cancer.
The immuno-oncology drug ‘Opdivo’ emerged as a first-line option, another immuno-oncology drug 'Keytruda' also obtained positive results.
A targeted therapy that targets a new protein is also expected to enter the market.
Two noteworthy studies in the field of gastric cancer were presented at the ‘2023 ASCO Annual Meeting (ASCO 2023)’ that was held for 5 days from the 2nd (local time).
The two were: results from the Phase III trial of ‘zolbetuximab,’ a targeted anticancer drug that targets CLDN18.2 (Claudin 18.2), and results from a Phase III trial that reviewed the first-line treatment of Keytruda in gastric cancer.
Claudin 18.2 is a protein mainly present on the surface of gastric cancer cells.
Although the protein is also present in normal cells, it is expressed at high levels in certain malignant tumors.
Claudin 18.2 is known to be involved in the proliferation, differentiation, and metastasis of cancer cells.
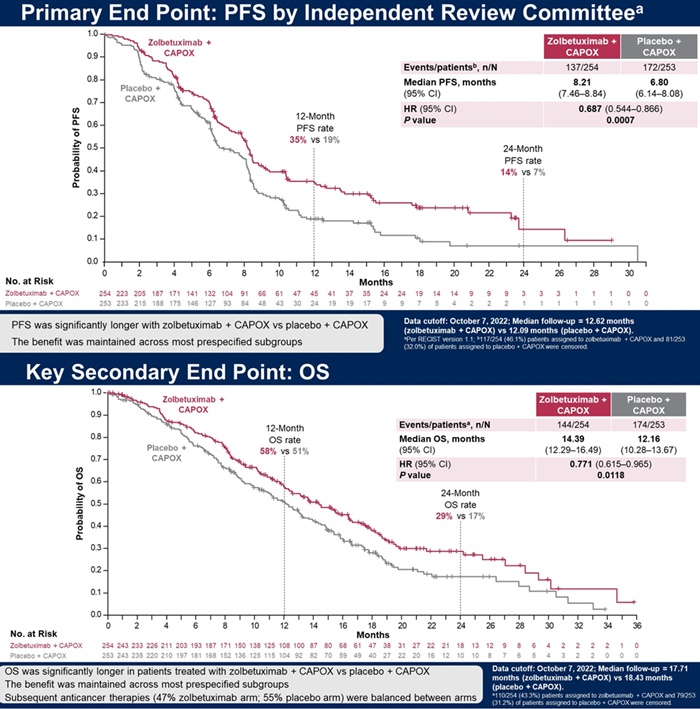
The GLOW clinical trial that was announced this year, compared zolbetuximab + CAPOX (capecitabine + oxaliplatin) combination therapy with just CAPOX in 507 patients with advanced·metastatic gastric cancer who were CLDN18.2 positive.
Of those involved, 78% had a PD-L1 combined positive score (CPS) less than 5.

At 24 months, the PFS rate in the zolbetuximab group was 14%, twice higher than the 7% in the placebo group.
The secondary endpoint, overall survival (OS) was 14.4 months in the zolbetuximab group and 12.2 months in the placebo group, demonstrating a 23% reduction in risk of death with zolbetuximab (HR=0.77).
In addition, the objective response rate was 53.8% and the duration of response was 6.3 months in the zolbetuximab group.
During an interview with Dailypharm, Min-Hee Ryu, Professor of Oncology at Asan Medical Center, said, “Zolbetuximab is expected to bring a positive effect as it has demonstrated superiority in OS as well as PFS.
In terms of side effects, although the frequency of nausea and vomiting, which was different from those of existing drugs, was high with zolbetuximab, but appears to be at a manageable level.
I believe the side effects arise because the targeted claudin protein is also present in normal cells.” Another noteworthy clinical trial is the Phase III KEYNOTE-859 that was conducted to evaluate Keytruda’s effect as a first-line treatment for gastric cancer.
This clinical trial is significant in that MSD succeeded in changing the design of the KEYNOTE-062 clinical trial, which had previously failed in the first-line, and was led by Korean medical staff.
The clinical trial compared Keytruda with the chemotherapy CAPOX or 5-FU (5-fluorouracil) to chemotherapy alone in patients with HER2-negative gastric cancer.
The trial also measured the therapy’s effect according to the patient’s PD-L1 CPS score.
The primary endpoint was overall survival (OS).
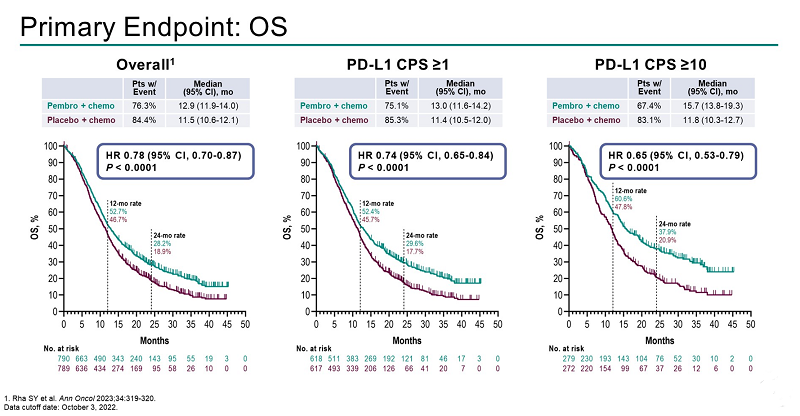
Keytruda’s effect increased with the increase in CPS.
The hazard ratio was 0.83 in the patient group with a CPS score of 1 to 10, and the hazard ratio was 0.64 in the patient group with a CPS score of 10 or more.
This means that in patients with a CPS score of 10 or higher, Keytruda lowered the risk of death by 35%.
In addition, the Keytruda group demonstrated significant efficacy in secondary key endpoints as well, including PFS.

Although Keytruda succeeded in demonstrating its effect later than Opdivo, it is meaningful as the results included PD-L1 CPS 1-4 point patients.
Sun-Young Rha, Professor of Oncology at Yonsei Cancer Hospital, said, “The fact that Keytruda showed an improvement with a hazard ratio of 0.83 even in patient groups that include patients with CPS 1 to 4, demonstrates that patients with a CPS of 1 or more may use the immunotherapy.” They agreed that it will become necessary to devise an effective treatment strategy according to the CPS score when zolbetuximab is introduced in the future.
Professor Ra said, "Our task for the future in gastric cancer is to reach a consensus on how to treat patients who are both CLDN18.2 positive and PD-L1 positive." Professor Rhu added, “There is some overlap between the areas for the use of zolbetuximab and immuno-oncology drugs.
in patients with CPS between 5 to 10, the effects of the two drugs are likely to be similar, so the side effect aspect should be considered.
For those with a CPS of 10 or higher, immuno-oncology drugs are definitely more effective."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









