- LOGIN
- MemberShip
- 2025-12-23 17:18:56
- Leclaza and Tagrisso compete for reimb in the first line
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-07-03 05:47:44
On June 30th, the Ministry of Food and Drug Safety approved the change in Leclaza’s indication to ‘the treatment of patients with advanced or metastatic non-small-cell lung cancer harboring EGFR mutation exon 19 deletions or exon 21 substitution.’ With the approval, Leclaza, which had been used as a second-line treatment until now, is now available as a first-line treatment in Korea.
As a result, 2 third-generation TKIs – Tagrisso and Leclaza – are now available for the treatment of EGFR-mutated NSCLC in the first line.
However, as the first-line indication for the drugs is yet to be reimbursed in Korea, the drugs are mainly used as subsequent therapy following initial treatment with first and second-generation drugs.
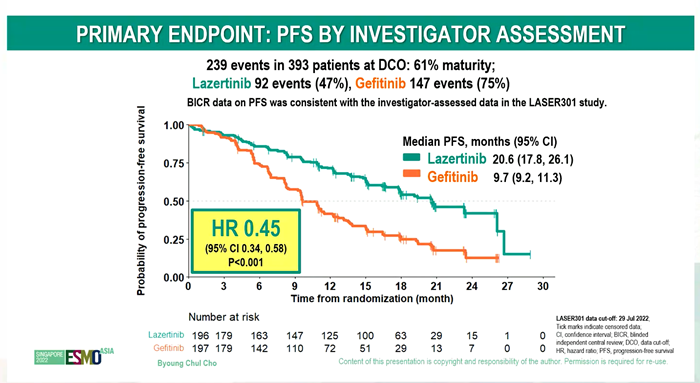
Also, Leclaza reduced the risk of disease progression and death by 55% compared to the control group (HR=0.45).
Also, its benefit was consistently observed in ▲patients with brain metastasis, ▲patients with L858R mutation, and▲ Asians.
Although a uniquely high incidence of paresthesia was observed in the Leclaza-treated group, most were mild and manageable.
◆Tagrisso and Leclaza compete for first-line reimbursement With the approval, Leclaza is now on a level playing field with Tagrisso.
Leclaza quickly closed the gap during the 4 years Tagrisso struggled and failed to pass its first step to receiving reimbursement in the first line.
Therefore, how the competition will end will now depend on which drug becomes reimbursed in the first line.
In the case of Tagrisso, its adequacy for reimbursement in the first line was finally recognized in March by the Health Insurance Review and Assessment Service’s Cancer Disease Deliberation Committee (CDDC) after 5 attempts.

The CDDC had deemed that the data was not sufficient to recognize the drug’s effect on Asians.
The company had strategically narrowed its reimbursement standards, but that attempt was also turned down by the CDDC.
The situation turned in favor of Tagrisso at the end of 2022 after large-scale real-world data on Tagrisso’s effect as a first-line treatment in Asia and Europe was released.
Analysis of the real-world data of 660 Japanese patients confirmed a progression-free survival of 20.0 months and overall survival of more than 3 years (40.9 months), which was longer than that found in its Phase III clinical trial.
Based on the data, the company put an end to Tagrisso’s efficacy controversy in Asia.
However, the problem is that the company has only now passed the first step to its reimbursement.
Tagrisso’s reimbursement agenda needs to pass HIRA’s Drug Reimbursement Evaluation Committee (DREC) review, drug pricing negotiations with the National Health Insurance Service, and the Ministry of Health and Welfare’s Health Insurance Policy Deliberation Committee (HIPDC) to complete the reimbursement process in Korea.
As a risk-sharing agreement (RSA) drug, Tagrisso must also pass pharmacoeconomic evaluations.
HIRA’s statuary evaluation period is set at 120 days or less, but it is common for HIRA to exceed the set deadline if the company is required to submit supplementary data.
In fact, 3 months have passed since Tagrisso passed the CDDC review, but no schedule for the subcommittee for its pharmacoeconomic evaluation has been set yet.
Although the statutory period set for the reimbursement process sets the timing for Tagrisso's reimbursement extension at the end of this year, there is a strong possibility that the period will be delayed somewhat.
Unlike Tagrisso, Leclaza’s reimbursement agenda is expected to pass CDDC review without difficulty as the drug demonstrated its effectiveness in the Asian subgroup with a hazard ratio of 0.46.
The fact that it is the only homegrown new drug is also expected to work in favor of Leclaza.
Therefore, if Leclaza passes the CDDC review in July or August, the drug may also be deliberated by DREC with Tagrisso.
Yuhan Corp plans to apply for reimbursement in the first line as soon as possible.
Also, the company has also prepared an Early Access Program (EAP) that provides Leclaza free of charge until the drug is granted reimbursement.
The move shows Yuhan Corp’s confidence that it will be able to rapidly receive reimbursement.
Yuhan Corp said, “With Leclaza’s approval, we are pleased to be able to provide a new treatment option for patients with EGFR mutation-positive NSCLC, which is highly prevalent in Korea.
We are preparing to apply for the reimbursement extension for Leclaza in the first line and provide our drug for free to the patients until it is reimbursed through our Early Access Program (EAP).”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









