- LOGIN
- MemberShip
- 2025-12-23 16:54:35
- Vabysmo’s real-world data to hold leader Eylea in check
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-07-06 05:39:48
Based on the real-world data that demonstrated Vabysmo’s consistent effect on patients who switched from Eylea, the company built evidence for patients to switch to Vabysmo.
To defend the lead, Eylea’s company has attempted to release a high-dose version of Eylea but has been experiencing difficulties due to its delayed introduction.
According to industry sources on the 5th, the global real-world data on Vabysmo was recently published in the international journal ‘Nature.’ The results of the investigator-led trial that was published are the first real-world data that provides a glimpse of what kind of effect Vabysmo can bring to the field..
Roche’s bispecific antibody Vabysmo (faricimab) is a new drug approved in Korea for the treatment of macular degeneration.
The current leader in this market is Bayer and Regeneron’s ‘Eylea (aflibercept).’ One thing to note was that a significant proportion of the patients included in the real-world study were those that had been previously treated with ‘Eylea.’ 337 of the 376 eyes of 335 patients that participated in the study had been previously treated with an anti-VEGF agent, 237 eyes of which were treated with Eylea.
Patients in the study switched to Vabysmo due to non-response or to extend their treatment cycle after using Eyelea.
The other 39 eyes were treatment-naive eyes.
The primary endpoints of the study were the changes in best-corrected visual acuity (BCVA), changes in central subfield thickness (CST), and safety, and the Secondary outcome measures included treatment intervals and the presence of retinal fluid.
Results showed that after a single injection of Vabysmo, the mean CST reduction in previously-treated eyes was -25.3μM, and this mean value became -26.3μm in patients who were previously treated with Eyela.
All patients treated with Vabysmo, including those with treatment-naïve eyes, demonstrated a mean reduction in CST of -31.3μm.
Also, a number of patients demonstrated complete resolution of intraretinal fluid (IRF), subretinal fluid (SRF), or pigment epithelial detachment (PED).
Patients demonstrated further improvement after 3 injections of Vabysmo.
In the 337 eyes that switched to Vabysmo, the mean BCVA increase from baseline was +2.7, and the mean CST reduction of -38.1μm.
Patients that switched from Eylea to Vabysmo demonstrated a mean BCVA increase of +2.2 letters, and a mean CST reduction of −42.6μ from baseline.
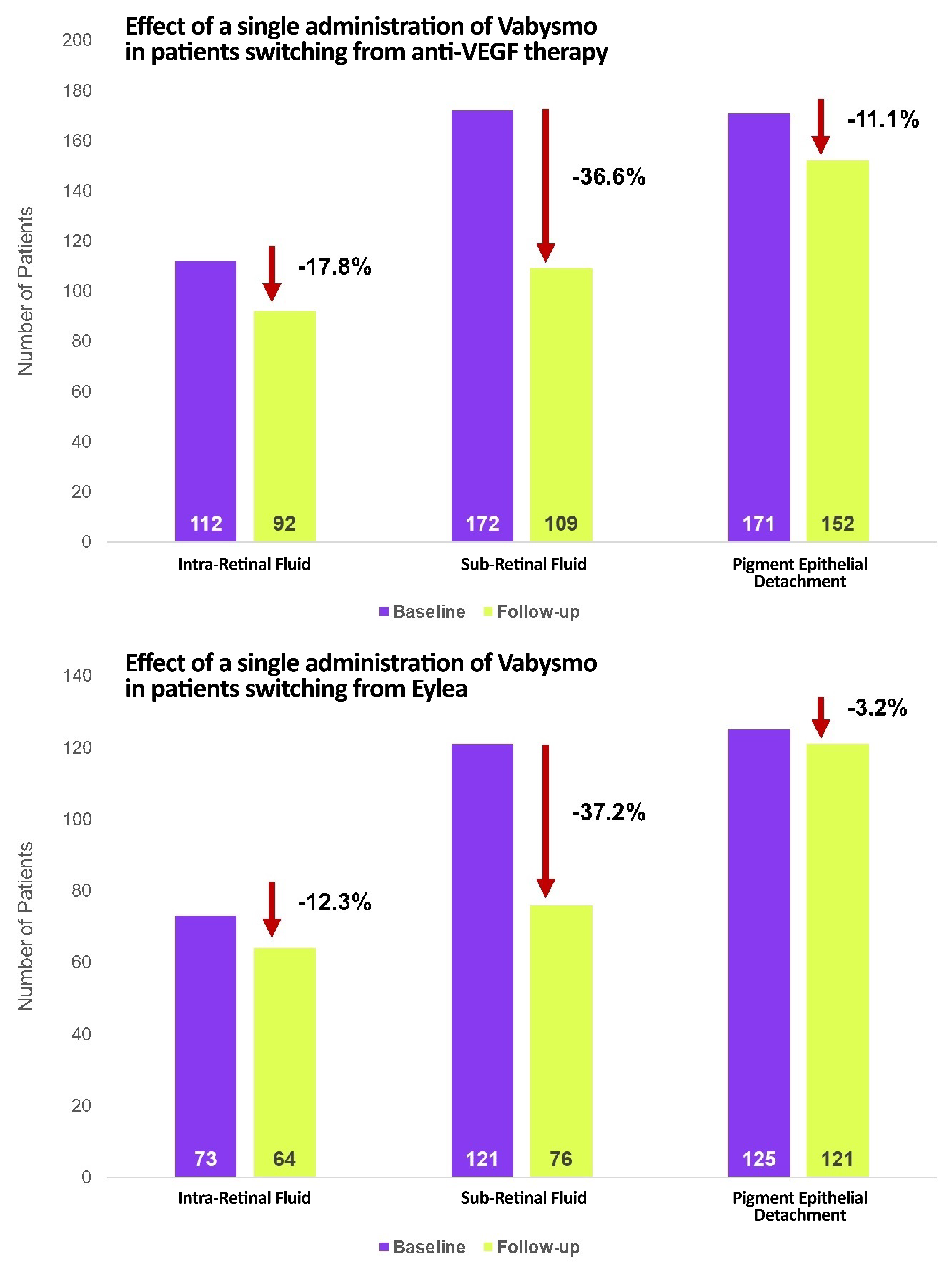
Patients that switched from Eylea showed a 12.3%, 37.2%, and 3.2% resolution of IRF, SRT, and PED, respectively.
The reduction or removal of retinal fluid from the use of Vabysmo is analyzed to have affected the maintenance and improvement of visual acuity.
Two cases of intraocular inflammations were reported in the 376 eyes treated in the study, and their vision returned to baseline after treatment.
◆ Eylea faces difficulties with Vabysmo chasing at its tail Based on the real-world results, Roche is expected to speed up targeting Eylea’s share.
Currently, Eylea has an overwhelming lead in the domestic macular degeneration treatment market.
According to the market research institution IQVIA, Eylea recorded annual sales of KRW 80.4 billion last year.
This is a 14% increase from 2021.
Eylea accounted for 64% of the total macular degeneration treatment market (KRW 126.3 billion).
Therefore, all the new drugs released to the market are targeting Eylea’s share, attempting to take a piece of the pie before Eylea’s patent expiry and the entry of its biosimilars.
In Korea, Vabysmo entered the market this year, following ‘Beovu’ in 2020.
In just 2 years of its release, Beovu posted KRW 16.5 billion in sales last year.
In particular, the new entrant Vabysmo has been considered a strong contestant against Eylea because after administering the initial 4 doses at 4-week intervals, and then, Vabysmo can be administered every 16 weeks (4 months) if there is no disease activity.
Many patients with macular degeneration often give up treatment due to the fear of receiving an injection in the eye, therefore extending the dosing interval was considered an important task in treatment development.
And the new contestant improved the convenience of patients with a 16-week dosing interval.
Eylea’s dosing interval can be extended up to 16 weeks if the patient’s disease is well managed, but the drug is administered every 4 weeks for the first 3 months and then every 8 weeks.
However, Eylea has the advantage of being able to flexibly take the treatment interval depending on the patient's condition, from 4 weeks to 16 weeks.
In the global market where Eylea and Vabysmo had already taken place, Vabysmo has been rapidly increasing its market share.
According to Roche's earnings report, Vabysmo’s global sales in Q1 this year were CHF 432 million (approximately KRW 620 billion).
When considering how the drug was approved in the US and Europe in January and September last year, respectively, the drug has shown high growth.
On the other hand, Eylea experienced a drop in sales for two consecutive quarters from Q4 last year.
Bayer and Regeneron had set out to introduce a high-dose 8mg version of Eylea to defend the market.
However, the companies are facing difficulties as the US FDA declined approval of the higher-dose version.
According to Regeneron, the approval was deferred due to a delay in the review of a drug’s third-party manufacturer and is not because of any efficacy or safety issues related to the drug.
As long as there is no problem with the drug, there is no possibility that the FDA will completely turndown its approval.
However, the FDA's decision delays the release of the high-dose version and is expected to delay Eylea’s defense strategy.
In Korea, the environment is still favorable for Eylea because Vabysmo has not been listed for reimbursement yet.
Therefore, Eylea’s sales this year will be affected by the timing of Vabysmo’s reimbursement listing.
Also, the growth of Beovu, which is being more actively used in Korea than in the global market, should be watched closely.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









