- LOGIN
- MemberShip
- 2025-12-23 16:53:55
- Release of triple-combo diabetes drugs imminent
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-07-07 05:43:43

However, unlike the 2-drug combos that are already in fierce competition even in the pre-release stage, industry interest in 3-drug combos is currently at best lukewarm.
The pharmaceutical industry pointed to the relatively challenging development environment for triple combination drugs as well as the impurity issues that exist.
Nevertheless, there are predictions that in the long term, the paradigm for antidiabetic combos will shift from dual therapy to triple therapy combinations.
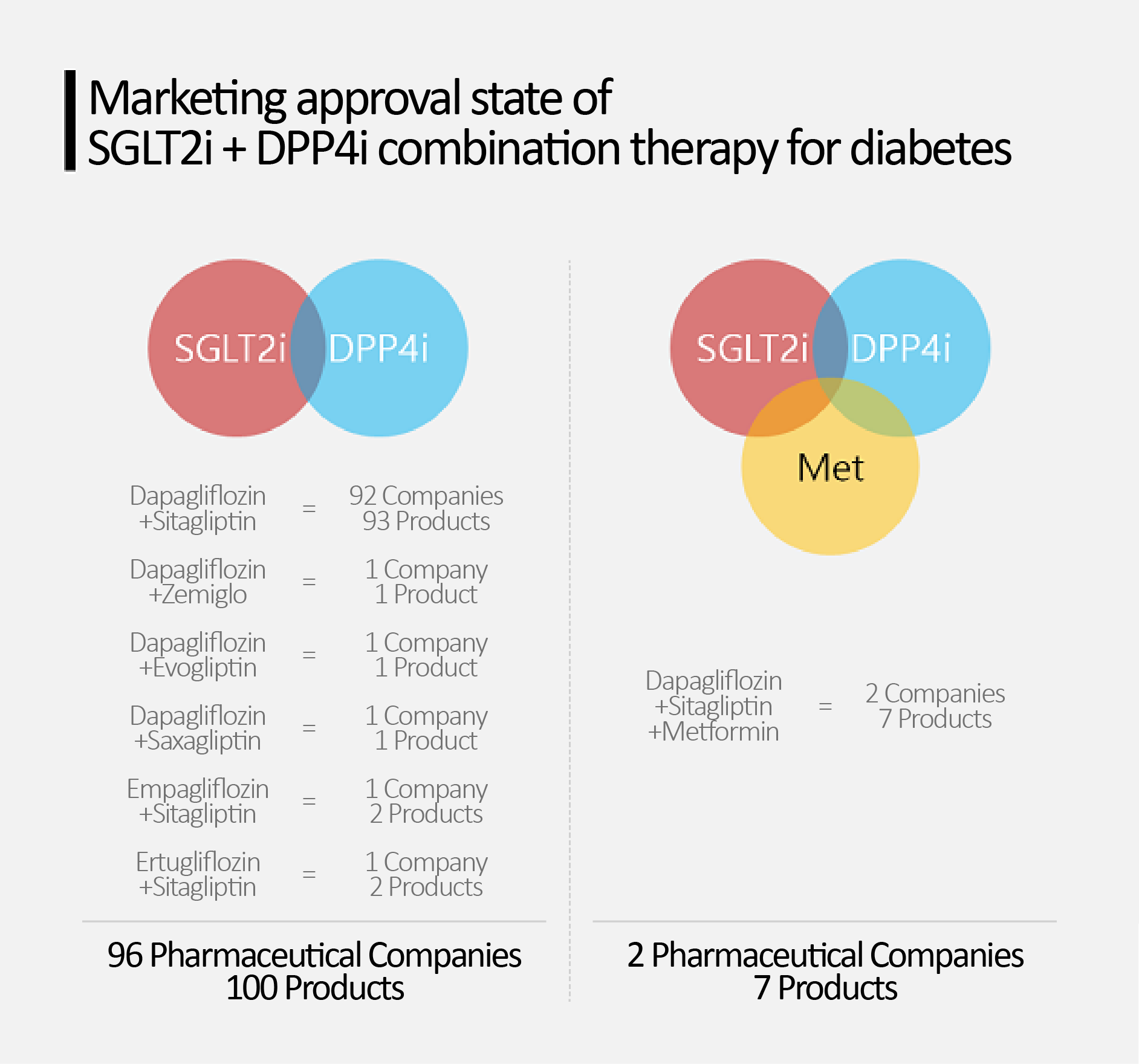
96 companies own 100 2-drug combos vs.
2 companies own 7 3-drug combos According to industry sources on the 6th, Daewon Pharmaceutical and Hanmi Pharmaceutical recently applied for the reimbursement of their 'dapagliflozin, sitagliptin, and metformin’ triple combination therapy for diabetes.
The drugs, Daewon Pharmaceutical's ‘Dapasita-M’ and Hanmi Pharm's ‘Sildapa M,' are expected to be released after September.
This is because the patent for the original sitagliptin drug Januvia will expire on September 1st.
Currently, the competition for antidiabetic combination drugs focuses on dual combinations.
As of the 5th, 96 pharmaceutical companies have received marketing approval for 100 dual combination products that combine SGLT-2 inhibitors and DPP-4 inhibitors.
This is in contrast to the fact that only 7 triple combination products from 2 companies have received marketing authorizations.
In the case of dual combinations, the dapagliflozin and sitagliptin combination accounts for the absolute majority with 93 products from 92 companies.
Many of these products are also expected to be released when the patent for Januvia expires.
While the interest in dual combination therapies is highly heated despite the possibility that a large amount of the products will be released at the same time, the attention towards triple combination therapies is still lukewarm.
In May, the government limited the reimbursement scope for diabetes treatment to combinations of ▲SGLT-2 inhibitors, DPP-4 inhibitors, and Mmtformin, ▲SGLT-2 inhibitors, TZD, and metformin, ▲Some SGLT-2 inhibitors and sulfonylurea or in combination with insulin.
The SGLT-2 inhibitor and DPP-4 inhibitor combination, which includes a majority of the dual combination therapies, was not included in the reimbursement scope.
When prescribing to patients, doctors have to add metformin to the dual combination therapy drugs to receive reimbursement.
"Difficult combine metformin due to its large dose… Impurity issues and concerns in development cost also exist" The analysis is that multiple factors have contributed to this phenomenon.
The relatively complex product development is considered the primary reason among them.
The dose of metformin, which is much larger compared to the other two drugs, posed an obstacle.
The dose of dapagliflozin is set to 5mg and 10mg, while sitagliptin is set to 50mg and 100mg.
However, the dose of metformin is set much higher at 500mg, 750mg, and 1000mg.
Technically, combining the three ingredients is not difficult.
However, due to the significant differences in dosages of each ingredient, the stability of the final product may be somewhat compromised.
There are three main methods of combining the three ingredients: single-layered tablet, double-layered tablet, and coating Metformin with DPP-4 inhibitors and SGLT-2 inhibitors.
Among these methods, dapagliflozin and sitagliptin can be combined relatively thinly in single- and double-layered tablets due to their small doses.
As a result, there is a higher possibility that the two ingredients may not interact adequately.
The method of coating metformin to the duo is not significantly different, but achieving a uniform thickness is difficult.
This process poses certain challenges, such as the need for repeated bioequivalence tests to verify the uniform pharmacokinetic action of all ingredients.
The impurity issue is also another factor that makes the development of triple combination therapy challenging.
Among the three ingredients in the combination therapy, impurities have been detected in metformin and sitagliptin.
As a result, when manufacturing pharmaceuticals with these ingredients, companies must validate whether or not impurities exist and submit one year’s worth of safety data to the Ministry of Food and Drug Safety.
The so-called ‘1+3 joint bioequivalence’ system is also considered a factor that makes product development challenging.
Under the Pharmaceutical Affairs Act that was implemented in 2021, the number of consignor pharmaceutical companies is limited to three per one consignee.
This imposes a higher cost burden on small and medium-sized pharmaceutical companies in the development process compared to the past.
An official from the pharmaceutical industry said, "Companies that started product development before the implementation of the regulation may be fine, but the potential burden for companies considering new development after that is substantial." They further explained, "In the past when 10 to 20 companies could share the cost, each company had a smaller burden.
However, we can now only recruit up to four companies, which imposes a larger cost burden for each company." Another official stated, “The guideline set by the Ministry of Food and Drug Safety for the bioequivalence testing of combination therapies have become more stringent, requiring more extensive clinical trials than before.
If the total cost for developing a combination therapy is around KRW 6 to 8 billion, each company should now bear approximately KRW 2 billion.
This is not an easy decision for small and medium-sized pharmaceutical companies to make." "In the long term, triple combination therapies will increase… Numerous companies have already started development” However, the predominant forecast is that the number of companies venturing into the development of triple combination therapies will increase in the long term.
This analysis is based on the fact that triple combination therapy is considered more advantageous than dual combination therapies in terms of convenience in taking the medicine and applicability for reimbursement.
An industry official said, “While it is considered more challenging compared to dual combination therapies, the development and manufacturing of triple combination therapies is not inherently difficult.
The market will move in the direction of reimbursement, to triple combination therapies.” Another industry official added, "As dual combination therapies are not eligible for reimbursement and require additional intake of metformin, the demand for triple combination therapies is expected to steadily increase in the future.
Apart from Daewon Pharmaceutical and Hanmi Pharmaceutical, several other companies have already started or are considering the development of triple combination therapies."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









