- LOGIN
- MemberShip
- 2025-12-23 17:16:42
- Will GC Pharma enter the US blood product market in 2013?
- by Chon, Seung-Hyun | translator Kwon Sung-Yong | 2023-07-19 00:28:14

For the past 13 years since it officially entered the US market in 2010, it has experienced growing pains such as failure to obtain permits and delays, but has attempted to enter the US market again.
According to the industry on the 18th, GC Pharma submitted a BLA for its immunoglobulin blood product ALYGLO to the US Food and Drug Administration (FDA).
ALYGLO applied for approval for primary immunodeficiency indication.
GC Pharma satisfied all efficacy and safety evaluation parameters according to FDA guidelines in the North American phase 3 clinical trial completed in 2020.
In the phase 3 clinical trial, ALYGLO was administered to 48 patients with primary immunodeficiency for 12 months, and the efficacy and safety were confirmed.
ALYGLO is a liquid-type immunoglobulin preparation purified from plasma fractions.
It is used to treat primary immunodeficiency diseases such as congenital immunodeficiency syndrome and immune thrombocytopenia.
According to GC Pharma, the US immunoglobulin market is estimated at about 12.5 trillion won.
With the recent increase in autoimmune diseases, the demand for immunoglobulins is continuously increasing.
GC Pharma explained, “The blood product, which requires large-scale facility investment and advanced production experience, is known to frequently have a supply shortage because producers are very limited.” It is the confidence that ALYGLO will be able to secure sufficient market competitiveness if it enters the US market.
This is the third attempt by GC Pharma to apply for FDA approval for a blood product.
GC Pharma applied for approval of the IVIG-SN 5% product to the FDA at the end of 2015.
FDA approval was expected at the end of 2016, but in November 2016, the FDA pointed out that the manufacturing process-related data should be supplemented.
In September 2017, GC Pharma's approval was delayed again due to a request for additional supplementation of manufacturing process data.
GC Pharma planned to enter the US market first with 5% products and later with 10% products undergoing clinical trials.
However, as the approval of the 5% product was delayed, the company changed its strategy to release the 10% product, which has greater marketability, to the US market first.
GC Pharma completed phase 3 clinical trials in North America for IVIG-SN10% ALYGLO in 2020 and submitted an application for product approval to the FDA in February 2021.
However, in February of last year, it received a notice from the FDA to postpone the approval of the product.
Due to the COVID-19 situation, a non-face-to-face evaluation was conducted in the fourth quarter of 2021, but the FDA decided to postpone the approval because of the need for an on-site inspection of the production facility.
From April 17th to 28th, the FDA inspection team conducted an inspection of production facilities and quality systems such as fractionation, stagnation, and finished products of IVIG-SN at GC Pharma's Ochang plant.
After completing the GMP inspection of the Ochang plant, GC Pharma resubmitted an application for permission after consulting with the FDA.
The FDA's product approval process goes through a preliminary review after receiving the BLA, sets a target date for completion of the review, and starts the review process in earnest if the data is appropriate.
A GC Pharma official said, “We are aiming to obtain product approval early next year and launch it in the US market in the second half of the year.” He said, “We will become a global leader in blood products based on our entry into the US market in the future.” If GC Pharma receives FDA approval for ALYGLO, it will enter the US market for the first time since its foundation.
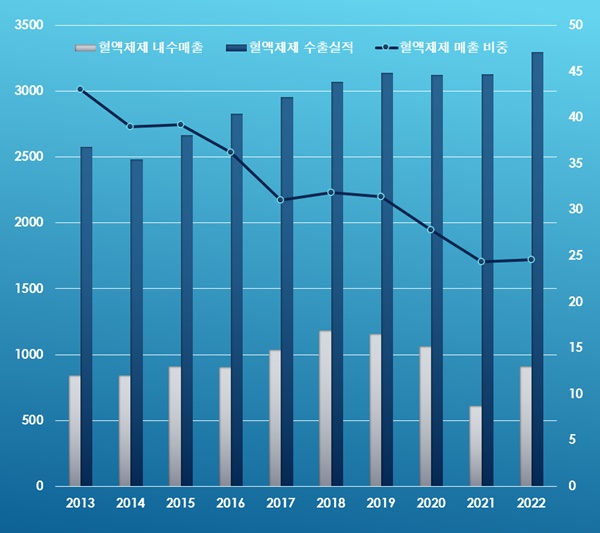
Last year, GC Pharma's blood product sales amounted to 420.4 billion won, accounting for 23.7% of the company's total sales.
GC Pharma's blood product sales grew only 12.4% over the past 9 years from 341.5 billion won in 2013.
Sales of blood products last year fell short of 429.6 billion won in 2019 and 425.4 billion won in 2018.
The share of blood products in GC Pharma's sales reached 43.0% in 2013, but fell to the 30% level in 2014 and fell to the 20% level from 2020.
Over the past nine years, the sales portion of blood products has declined by 19.4%.
Blood product export growth is slow.
Last year's GC Pharma blood product export performance was 90.9 billion won, up 48.3% from the previous year.
However, considering that blood product exports recorded more than 100 billion won for four consecutive years from 2017 to 2020, a breakthrough in overseas markets is urgently needed.
GC Pharma recorded 90.9 billion won in exports of blood products last year.
If ALYGLO enters the US market, the proportion of overseas sales is expected to increase.
In 2010, GC Pharma announced the entry of blood products into the US market.
GC Pharma signed a contract with ASD Healthcare in 2010 to export blood product IV Globulin SN and hemophilia treatment GreenGene F worth a total of $480 million over three years.
However, when the clinical trial period was delayed than originally planned, the memorandum of understanding with ASD Healthcare was also terminated in September 2015.
GC Pharma also faced setbacks in its hemophilia treatment plan to enter the US market.
In October 2016, GC Pharma decided to suspend the US clinical trial of GreenGene F, a recombinant hemophilia A treatment that is undergoing phase 3 clinical trials in the US.
In 2012, it announced its withdrawal after 4 years of entering phase 3 clinical trials.
The reason for the discontinuation of clinical trials in the United States was pointed out as “deterioration in business feasibility.” Due to the nature of rare diseases, the recruitment of new patients was slow, leading to delays in clinical trials and the emergence of competitive drugs with long duration of action. Due to GC Pharma's delay in entering the US market, the blood product supply strategy has also changed.
Initially, GC Pharma sought to enter the blood product market through a local plant in North America.
Green Cross Holdings spent 210 million Canadian dollars (about 187 billion won) in 2017 to complete the blood product plant in Montreal, Quebec, Canada.
The plant, built on a land area of 63,000 square meters, has a process for producing blood products such as IV Globulin and albumin by fractionating up to 1 million liters of plasma per year.
However, the North American subsidiary was liquidated as the US approval of IVIG-SN, a blood product, was delayed more than expected. In July 2020, Green Cross Holdings sold two of its North American blood products affiliates to Spain's Grifols, the world's largest blood products company, for a total of $460 million.
GCBT (Green Cross BioTherapeutics), a subsidiary of Green Cross North America (GCNA), was sold for 189.1 billion won, while another US subsidiary, GCAM (Green Cross America), was also handed over.
GCBT is a blood derivatives plant built by GC in Canada.
GCAM is a corporation that supplies plasma in the US.
It has 12 blood centers in the US.
Initially, a structure in which GCBT would produce blood derivatives with raw plasma made from blood secured by GCAM was conceived, but the business strategy was drastically revised in consideration of the uncertainty caused by changes in business conditions.
In the case of GCBT in Canada, although facility investment has been completed, it has been receiving manpower and technical support from GC Pharma's headquarters for commercial operation since 2018 due to a lack of local bio-production process experts.
In this situation, as Grifols actively sought out the acquisition, the sale was carried out in a flash.
GC Pharma is said to have recovered most of its investments in GCBT and GCAM.
If ALYGLO obtains FDA approval, it will be produced at GC Pharma's Ochang plant and sold through GC Biopharma USA, a US GC Pharma subsidiary.
A GC Pharma official said, "Immune globulin use in the United States is expected to increase further due to the increase in autoimmune diseases due to the aging population and improved awareness of the diagnosis and treatment of congenital immunodeficiency syndrome." “We expect to improve the situation of limited product supply,” he predicted.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









