- LOGIN
- MemberShip
- 2025-12-23 17:15:40
- Power of K-combos...Rosuzet leads outpatient Rx drug market
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-07-19 05:20:12
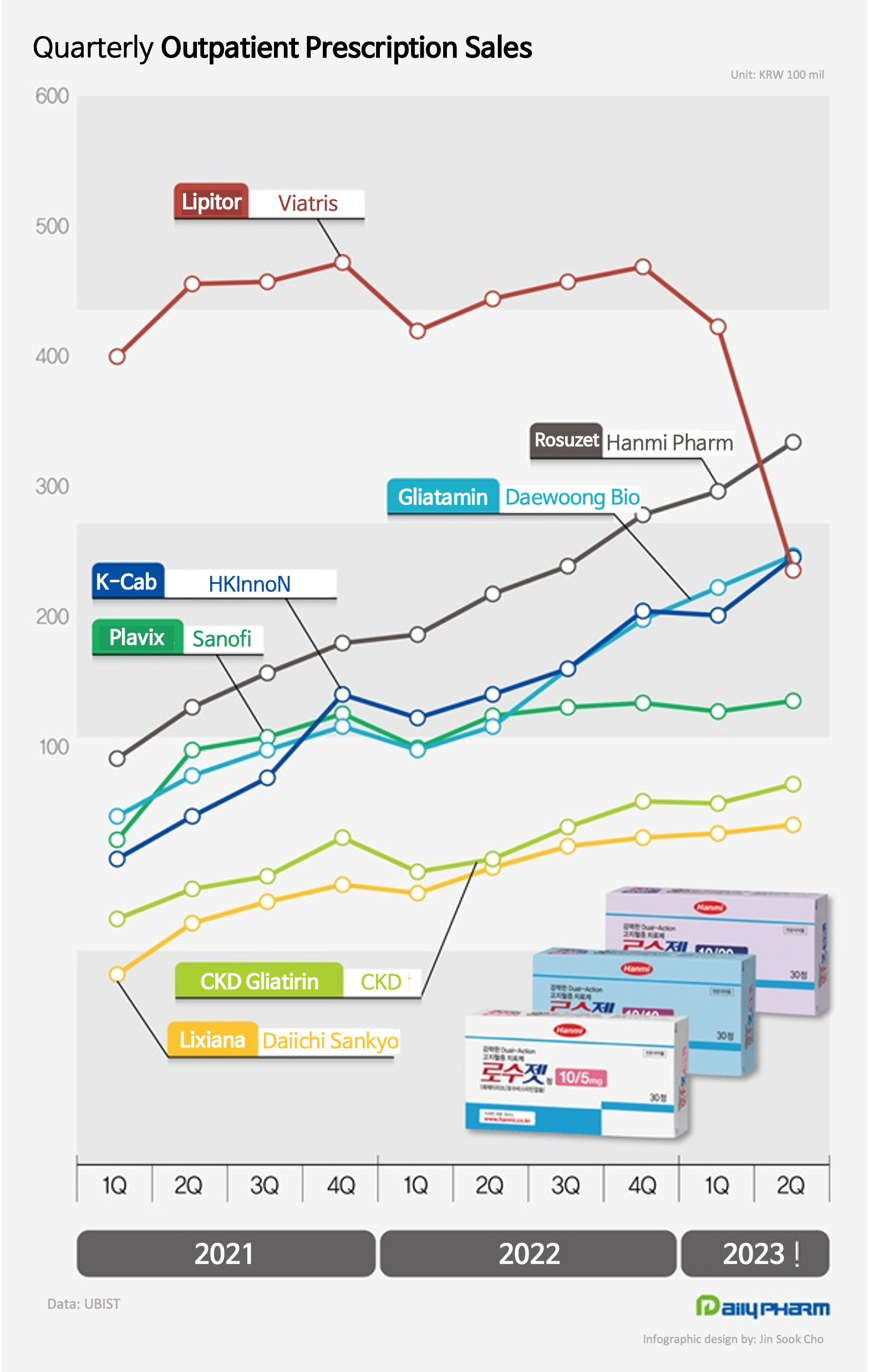
Hanmi Pharmaceutical’s new combination drug Rosuzet is ruling the outpatient prescription market in Korea.
Rosuzet became the first domestically developed drug to lead quarterly outpatient prescriptions sales.
Sales of HK Inno.N’s new drug ‘K-cab’ also continued to grow, taking part in the lead owned by domestically developed drugs in the outpatient prescription market.
According to the market research institution UBIST, Hanmi Pharmaceutical’s Rosuzet recorded the most in outpatient prescriptions in Q2 with KRW 43.8 billion.
Its sales rose 19.5% YoY and overtook the lead in quarterly prescriptions for the first time.
Viatris’s Lipitor, which had held the lead until Q1 last year, was overtaken for the first time.
By monthly prescriptions, Lipitor’s monthly prescriptions had exceeded Rosuzet’s by KRW 300 million until April.
At the time, Lipitor’s posted sales of KRW 14.1 billion and Rosuzet KRW 13.8 billion.
However, Rosuzet beat Lipitor (KRW 12.6 billion) by KRW 2.2 billion, posting KRW 14.8 billion in sales from May.
Rosuzet maintained a KRW 2.1 billion sales gap with Lipitor with prescription sales of KRW 15.2 billion in June.
Rosuzet, which was released at the end of 2015, is a combination drug for hyperlipidemia that contains rosuvastatin and ezetimibe.
Rosuzet has been continuing rapid growth in the market benefitting from its preoccupation of the market and the rising popularity of the statin and ezetimibe combination.
Rosuzet’s prescription sales rose twofold in 3 years from KRW 24.9 billion in Q2 2020, continuing strong growth.
Rosuzet’s sales exceeded KRW 100 billion for 3 consecutive years from 2020 and reserved the ‘KRW 100 billion sales club’ for the 4th consecutive year by making KRW 85.3 billion in 1H this year.
Until this year, Lipitor had never lost its lead.
Lipitor maintained its lead until Q1 this year, posting sales of KRW 49.2 billion, which is KRW 7.7 billion more than the second-runner Rosuzet.
However, in Q2 this year, prescriptions had dropped 25.1% YoY and fell to 4th place.
In 1H, Lipitor recorded KRW 87 billion in cumulative outpatient prescription sales, slightly ahead of Rosuzet (KRW 85.3 billion).
Lipitor, which was introduced to the domestic market in 1999, is an atorvastatin-based dyslipidemia treatment.
Although it continued to exert a strong influence in the prescription drug market despite the entry of its generics after patent expiry, its growth slowed down recently.
HK Inno.N’s new drug K-cab has also continued to make strong sales.
K-cab posted sales of KRW 38.5 billion in Q2, up 19.9% YoY and ranking 3rd in outpatient prescriptions.
K-cab, which was released in March 2019, is a new -CAB (potassium-competitive acid blocker) class drug for gastroesophageal reflux disease (GERD).
It has a new mechanism of action that inhibits gastric acid secretion by competitively binding to the proton pump and potassium ion located in the final stage of acid secretion.
K-cab exceeded KRW 100 billion in prescriptions in its 3rd year of release in 2021 and recorded sales in the KRW 100 billion range for 2 consecutive years.
K-cab made prescriptions of KRW 74.1 billion in the 1H this year, also heralding a record that exceeds KRW 100 billion won for three consecutive years.
In addition to being approved for the treatment of erosive and non-erosive GERD, then gastric ulcer, K-cab acquired additional indications as an antibiotic combination therapy for the eradication of Helicobacter pylori in patients with peptic ulcer and/or chronic atrophic gastritis, and as maintenance therapy after treatment of GERD.
Initially, the drug was granted reimbursement for the GERD and gastric ulcer indication among the 5, and was additionally granted reimbursement for the other indications.
Therefore, K-cab's sales growth is expected to continue to increase further.
Among domestically developed new drugs, Daewoong Bio’s brain function enhancer Gliatamin’s prescriptions rose 26.0% YoY in Q2 to record KRW 38.5 billion and rank 2nd in outpatient presciptions.
Gliatamin’s increased its influence in the prescription market despite difficulties faced due to narrowed reimbursement standards, controversy over its efficacy, and the government's order to initiate negotiations to redeem the reimbursed claims amount.
Chong Kun Dang Gliatirin, which also contains choline alfoscerate, also continued its high march in Q2, up 14.5% YoY and posting KRW 27.8 billion in prescription sales .
Daiichi Sankyo’s anticoagulant Lixiana also sold KRW 25.9 billion in Q2 this year, an 8.6% increase YoY, and ranked among the top.
Lixiana’s sales in 1H were KRW 52.4 billion and are expected to exceed KRW 100 billion for the first time in annual prescription sales this year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









