- LOGIN
- MemberShip
- 2025-12-23 17:05:20
- Prescription of HA eye drops record KRW 166 bil in 1H
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-08-07 05:23:02

In 1H alone, the market grew exceeded KRW 160 billion in prescriptions this year.
The number of companies with half-year prescriptions that exceed KRW 10 billion increased from 5 in 1H last year to 7.
The pharmaceutical companies that will inevitably suffer huge losses due to the expected reimbursement re-evaluations are preparing to jointly respond to the government's measures.
Prescription of HA eye drops exceed KRW 160 bil in 1H, 7 companies exceed KRW 10 bil According to the market research institution UBIST, outpatient prescription of HA eye drops in 1H this year was KRW 16.6 billion.
This is a 16% increase from 1H last year.
This market shrunk somewhat in 2020 due to the spread of COVID-19 but has been rapidly expanding since then.
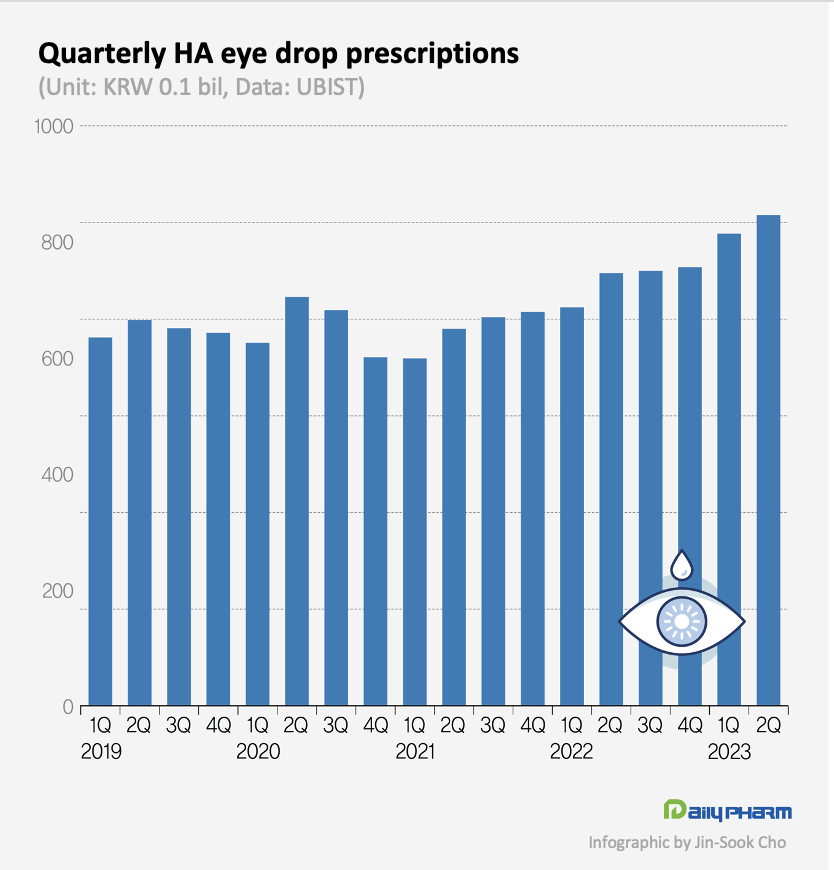
At this rate, the total market size may increase to exceed KRW 300 billion this year for the first time this year.
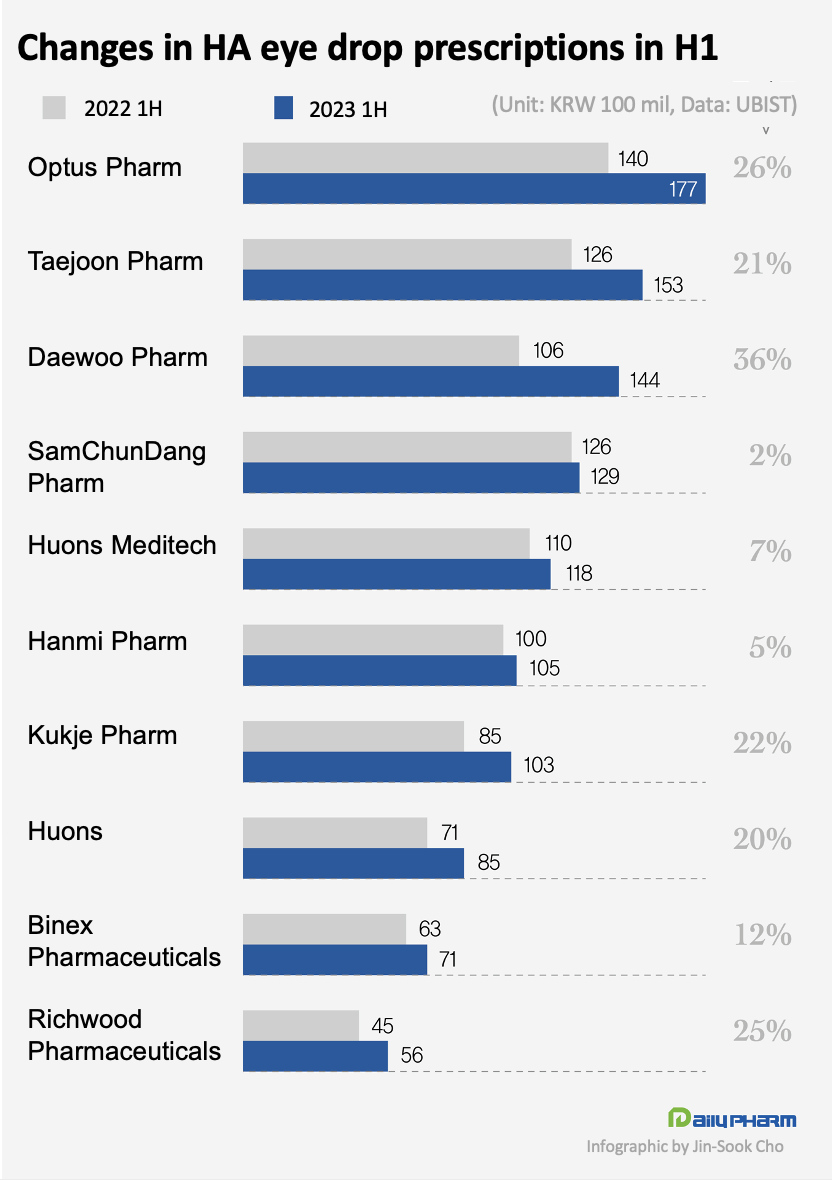
The prescription performance of major companies in the market has also increased significantly.
The prescription performance of Optus Pharm, which owns the Tearin series, increased by 26% from KRW 14 billion in 1H last year to KRW 17.7 billion in 1H this year.
In the case of Taejoon Pharm, which owns New Hyalyuni, Hyalyuni, and New Hyaldrops, its sales increased by 21% from KRW 12.6 billion to KRW 15.3 billion.
Sales at Daewoo Pharm, which owns Hyalsan, rose 36% from KRW 10.6 billion to KRW 14.4 billion.
As a single product, Hyalsan recorded the highest prescription performance in 1H this year.
The number of companies whos half year sales exceeded KRW 10 billion increased from 5 in 1H last year to 7 in 1H this year.
As of 1H this year, Optus Pharm, Taejoon Pharm, Daewoo Pharm and SamChunDang Pharm, Huons Meditech, Hanmi Pharm, Kukje Pharm recorded prescriptions of more than KRW 10 billion.
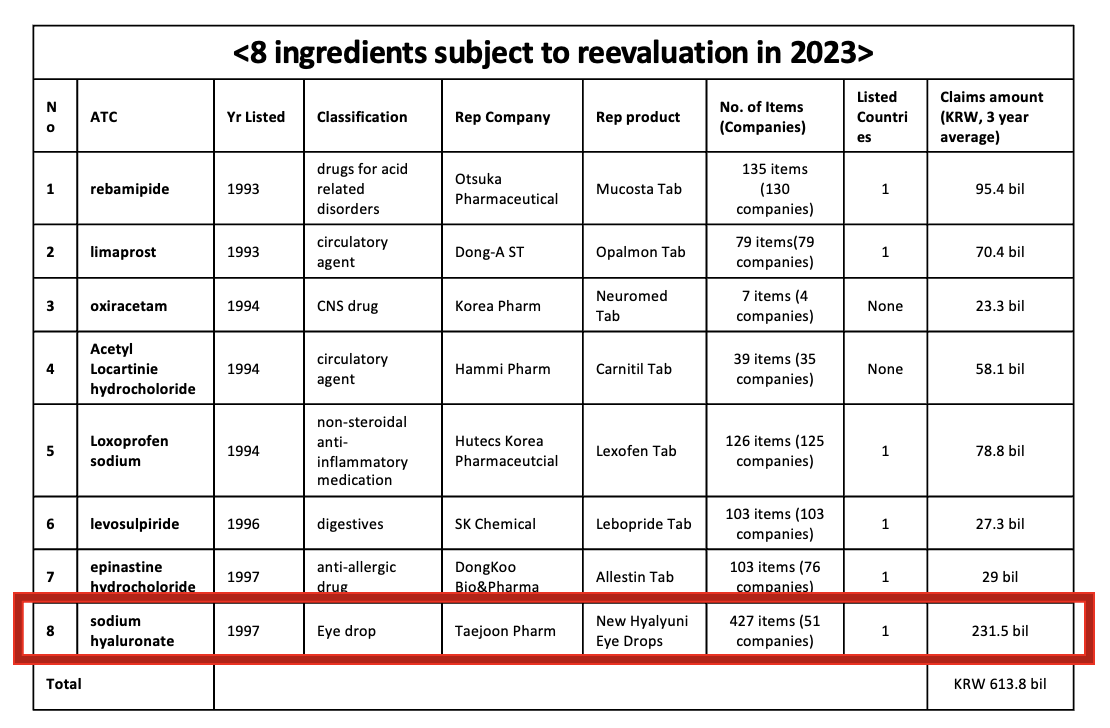
The government has selected 8 ingredients, including HA eye drops, as targets for reimbursement revelations this year.
These include rebamipide, limaprost oxiracetam, acetyl Locartinie hydrochloride, loxoprofen sodium, levosulpiride, epinastine hydrochloride, and HA eye drops Among them, oxiracetam and acetyl L-carnitine hydrochloride are expected to fail to pass the reimbursement revelations as they failed to pass the clinical reevaluations conducted by the Ministry of Food and Drug Safety.
Among the 8 ingredients, HA eye drops have the largest scale in terms of number of items and market size.
46 pharmaceutical companies have been selling 77 HA eye drop products in 1H this year.
The government has already received related data from each pharmaceutical company and started the practical review.
The outline of HA eye drops’ adequacy of reimbursement will be revealed next month at the earliest.
After holding an objection period for the pharmaceutical industry, the final result of the reimbursement reevaluation will be determined by the end of this year.
This is why the companies have been jointly preparing a response to the reevaluation results.
About 10 companies, centered around Optus Pharm, have been concentrating on developing a countermeasure with large law firms in Korea.
As in the case of choline alfoscerate, there is a possibility that the companies will file a class action lawsuit against the government.
If they do file a class action lawsuit, the suit will likely be subject to the drug expenditure refund and redemption law that passed the National Assembly this year.
The law, which will be enforced on November 20th, allows for the recovery or refund of drug costs that were already paid according to the outcome of the lawsuit if a pharmaceutical company delays government actions such as drug price cuts by applying for a suspension of execution along with an administrative lawsuit.
Some companies have also started to develop alternatives in preparation for the market withdrawal of HA acid eye drops.
In June of last year, Samil Pharmaceutical and Kukje Pharm received permission for Reba-K eye drops and Reba-i eye drops that contain rebamipide to treat dry eye syndrome.
Daewoo Pharm is also known to be developing an eye drop product with the same ingredient.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









