- LOGIN
- MemberShip
- 2025-12-23 17:15:58
- Immuno-anticancer drugs have brought us closer
- by Jung, Sae-Im | translator Kim, Jung-Ju | 2023-08-08 05:30:25
Following 'Opdivo (Nivolumab),' which obtained the indication for adjuvant therapy before surgery for the first time, 'Keytruda (Pembrolizumab)' is also seeking to expand its scope as adjuvant therapy before and after surgery.
The data shown by immuno-anticancer drugs in early lung cancer were surprising.
When chemotherapy and CCRT were used, 'pCR', which was seen in 1 or 2 out of 100 patients, increased more than 10 times.
In the case of Opdivo, a pCR of 24% was recorded in CheckMate-816 phase 3, significantly improving the 2.2% of the chemotherapy alone group.
At 3 years, EFS was 57%, exceeding the control group's 43%.
Keytruda also had an event-free survival rate of 62.4% at 2 years, which was significantly higher than that of the control group, 40.6%.
The mPR and pCR ratios were 30.2% and 18.1%, respectively, demonstrating a greater improvement than the control group of 11% and 4%.
Lee Se-hoon, a professor of hemato-oncology at Samsung Seoul Hospital, said in a recent interview with Daily Pharm, "If Opdivo caused a 'frenzy' as an adjuvant therapy before surgery, Keytruda gave the answer that it is helpful for patients to use immuno-oncology for a certain period after surgery." “We are now able to take a step closer to the goal of complete cure with immuno-oncology,” he said.

Professor Lee explained, "When Gefitinib, the first EGFR-targeted anti-cancer drug, came out as a fellow in the past, I was very surprised that the prognosis of patients improved significantly.
I am feeling the emotions I felt then once again as I look at adjuvant therapy before and after immuno-anticancer drug surgery." He said, "The professors of the Department of Pathology were also surprised to see that there were so many 'cancer-free' cases when they did a PET scan after immuno-anticancer treatment.
Not only did the pathological CR increase, but I felt that the overall response improved." If Opdivo showed significant improvement in adjuvant therapy before surgery for the first time, Keytruda could improve the prognosis even for patients who have not reached complete pathological remission if they use immuno-anticancer drugs for one more year as adjuvant therapy after surgery.
Proven.
While the Opdivo study used only adjuvant therapy before surgery and there was no follow-up treatment after surgery, Keytruda's KEYNOTE-671 study used up to 4 cycles of Keytruda + chemotherapy on patients before surgery and performed Keytruda alone after surgery.
The design was designed to use up to 13 cycles of therapy.
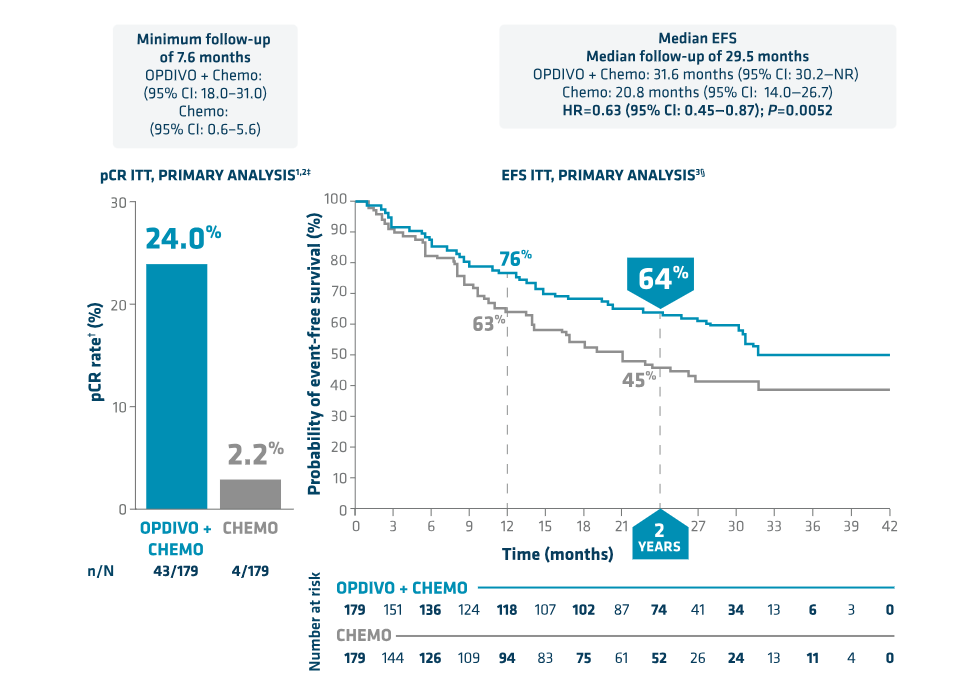
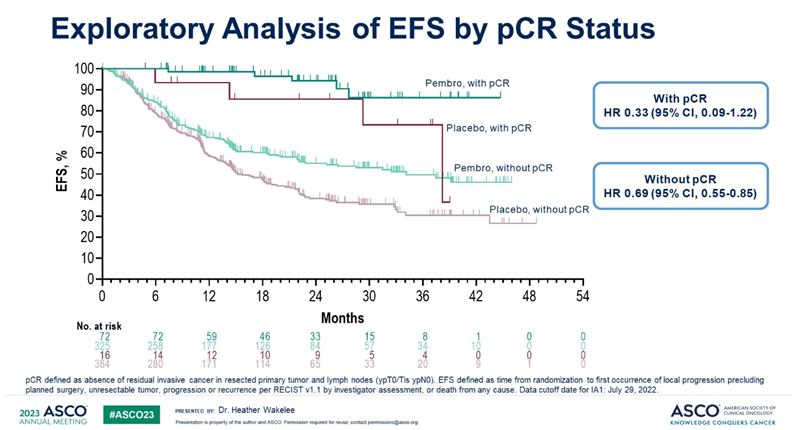
Patients who did not achieve pCR by administering immuno-anticancer drugs before surgery and administered Keytruda after surgery reduced the risk of disease recurrence, progression, or death by 31% compared to the control group.
Professor Lee said, "I knew that immunotherapy before surgery would be of great help from the Opdivo study, but there was no answer for postoperative treatment.
Because the clinical design was designed to use Opdivo only as an adjuvant therapy before surgery, Treatment was a situation where clinicians had to take care of themselves, so everyone was interested in how to treat patients who did not achieve complete remission.” The Keytruda study provided answers to questions raised in the clinical field.
Professor Lee said, “I was convinced through the KEYNOTE-671 study that the use of immuno-anticancer drugs after surgery is helpful even in the group that did not show pCR,” and “If you look at the EFS graph, the group that did not achieve pCR used Keytruda.
You can see that the graph is clearly wider than the group that did nothing."
Representative examples include use in patients with major mutations such as EGFR and ALK, and screening of patients who may experience difficulty in surgery with little improvement when using immuno-anticancer drugs before surgery.
However, he said, it is self-evident that immuno-anticancer drugs are being used for early lung cancer and are getting closer to the goal of a complete cure.
Professor Lee said, "The title of the 'ASCO' session, which included the KEYNOTE-671 research presentation, was 'The Promise of Neoadjuvant Immunotherapy Across Solid Tumors'.
As in the usual case, this study was not included in the lung cancer session.
Here, preoperative adjuvant immunotherapy for lung cancer You can notice the meaning of therapy.
It means that immuno-anticancer drugs have begun to actively intervene in the goal of complete recovery with adjuvant therapy before surgery.
I am happy to think that it has shown a major change in the cancer treatment paradigm."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









