- LOGIN
- MemberShip
- 2025-12-23 13:23:04
- Released next-generation growth hormone drug
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-08-31 05:25:44
The domestic growth hormone drug market, which is rapidly growing, has faced a variable with the release of new products by major companies.
LG Chem, the No.
1 company in the market, replaced the existing Eutropin with the next-generation product, Eutropin S, further solidifying its leading position, followed by Dong-A ST and Pfizer.
In particular, Pfizer plans to add NGENLA, a once-a-week medication, to its lineup starting next month.
Depending on the success of this product, considerable changes are expected in the growth hormone market in the future.
Expected to exceed 250 billion won in the growth hormone market, successful replacement of LG Chem’s Eutropin S generation.
According to IQVIA, a pharmaceutical market research institute, on the 29th, the size of the domestic growth hormone drug market in the first half of last year was 135.6 billion won.
It increased by 18% from 114.6 billion won in the first half of last year.
The domestic growth hormone drug market is rapidly growing in recent years.
The market, which was worth 145.7 billion won in 2019, exceeded 150 billion won in 2020 and 200 billion won in 2021.
Last year, it further expanded to 238.6 billion won.
In the case of this year, it recorded 130 billion won in the first half alone and is expected to expand to more than 250 billion won by the end of the year.
It was found that related sales of major companies increased together.
LG Chem's combined sales of Eutropin and Eutropin S increased by 19% in one year from 42.1 billion won in the first half of last year to 50 billion won in the first half of this year.
LG Chem received permission for Eutropin S in April last year.
Then, at the end of last year, sales began in earnest.
Compared to existing products, the expiration date has been increased from 18 months to 24 months.
LG Chem explains that the expiration date has been extended, improving supply stability and making it possible to check the remaining capacity.
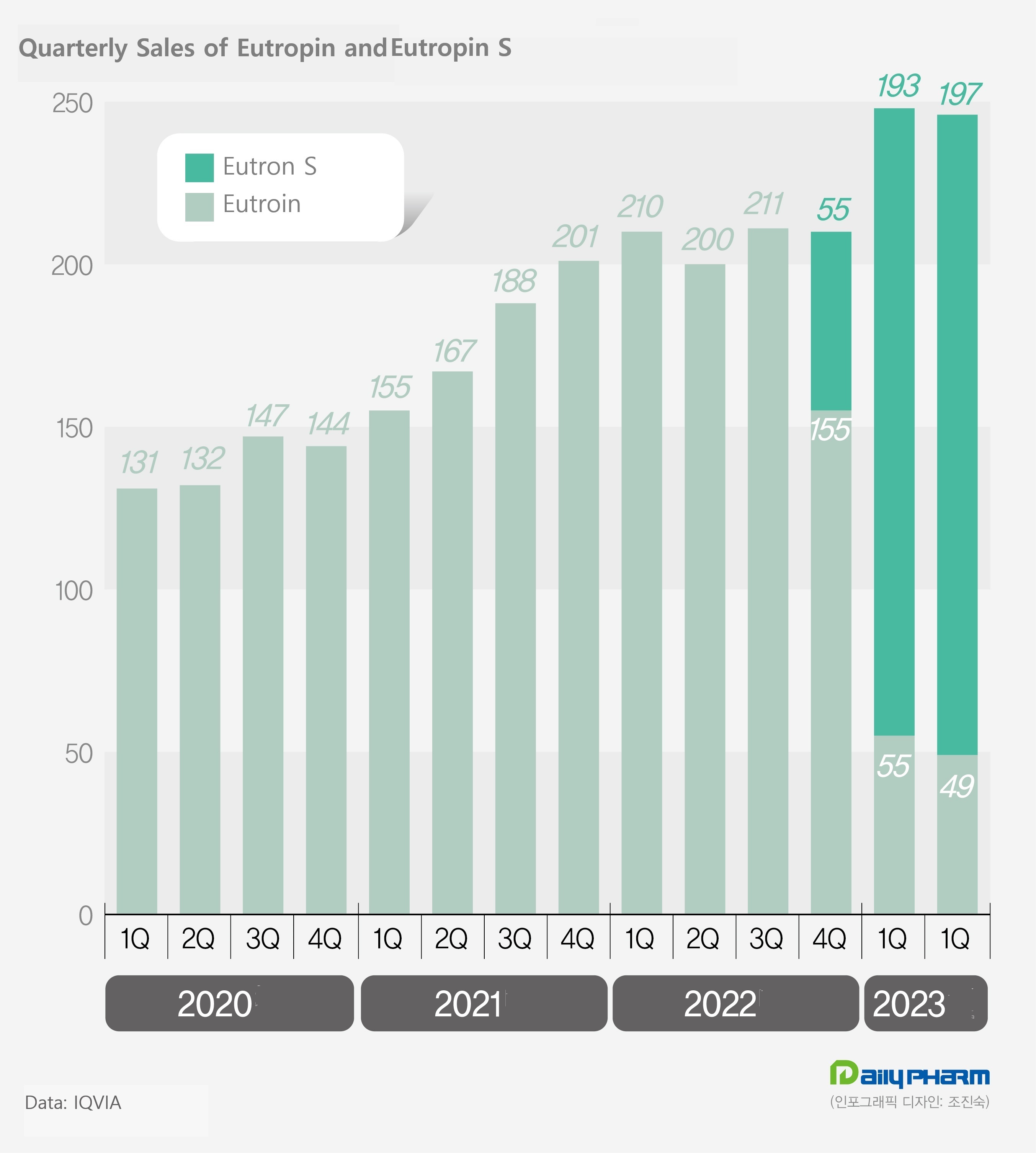
Sales of Eutropin S increased from 5.5 billion won in the fourth quarter of last year to 19.3 billion won in the first quarter of this year and 19.7 billion won in the second quarter.
Existing Eutropin sales decreased to 15.5 billion won, 5.5 billion won, and 4.9 billion won during the same period.
In the case of combined sales, it has been on an upward trend since the addition of new products.
Pfizer, No.
3 in the market, announces the release of NGENLA once a week.
LG Chem's Eutropin/Eutropin S, Dong-A ST's Growtropin II, and Pfizer's Genotropin/Genotropin goquick pen are rapidly catching up.
In particular, the increase in related sales of the two companies was found to be greater than that of LG Chem, the market leader.
Dong-A ST's Growtropin II posted sales of 33.2 billion won in the first half.
Compared to 21.2 billion won in the first half of last year, it increased by 57% in one year.
Growtropin II, which exceeded 10 billion won in quarterly sales in the first quarter of 2022, exceeded 15 billion won in sales in the first quarter of this year, and quarterly sales are about to reach 20 billion won.
Pfizer's Growtropin series is showing even greater sales growth.
The combined sales of Growtropin and Genotropin goquick pen increased by 86% in one year from KRW 14.1 billion in the first half of last year to KRW 26.3 billion in the first half of this year.
Genotropin, a cartridge-type product, increased by 48% from KRW 8 billion to KRW 11.8 billion, and Genotropin goquick pen, a pen-type product, increased 2.4 times from KRW 6.1 billion to KRW 14.5 billion.
It is analyzed that sales of Genotropin goquick pen, a next-generation product, are gradually expanding as the domestic supply of the two products stabilizes after 2020.
Pfizer plans to add a new product to its lineup.
Pfizer received permission for NGENLA Prefilled Pen Injection in January of this year.
This product will be applied for reimbursement from next month.

If the existing Genotropin was administered daily, NGENLA is administered once a week.
However, the indication is limited to the treatment of growth failure in children (3 years of age or older) due to pituitary growth hormone secretion disorders.
Pfizer plans to continue supplying Genotropin even if a new treatment is released, as NGENLA has a smaller indication range than Genotropin.
In addition, Merck's Saizen increased sales by 27% from 16.1 billion won in the first half of last year to 20.4 billion won in the first half of this year.
Sales of Novo Nordisk Norditropin nordiflex plunged from 16.3 billion won in the first half of last year to 1.1 billion won in the first half of this year.
Norditropin is known to have been discontinued in Korea since the third quarter of last year.
The company initially announced the timing of domestic resupply at the end of November last year but corrected it to say that it is currently undecided.
In the first half of the year, sales of Cyzen Korea Scitropin decreased by 8% from 3.8 billion won to 3.4 billion won, and Ferring Zomacton's sales increased from 1.1 billion won to 1.2 billion won.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









