- LOGIN
- MemberShip
- 2025-12-23 09:54:02
- Chong Kun Dang receives attention for its 'R&D potential'
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-12-08 17:28:26
Chong Kun Dang Pharmaceutical (CKD Pharm) has reported over 10 billion won of its R&D costs as assets for the first time.
The cost includes increased clinical trial costs for the commercialization of its biosimilars and incrementally modified drugs (IMD).
The company has gained recognition for its R&D potential following a major technology export recently, which has drawn significant attention to its drug development pipeline.

In 2019, the FSS issued a guideline that only R&D projects with technical feasibility such as new drugs could be accounted for as assets.
FSS’s R&D cost capitalization guideline set was the initiation of Phase 3 clinical trials for new drugs and approval of Phase 1 clinical trials for biosimilars.
As for generics, approval of bioequivalence test plans.
CKD’s intangible R&D assets were only 3.8 billion won at the end of last year.
This year, it has rose to 6.6 billion won, increasing by 2.8 billion won in Q1 and 2.8 billion and 2.2 billion won in Q2 and Q3, respectively.
With its drug development pipeline nearing commercialization, more R&D costs were reported as assests.
The company's combination drugs contribute to a big part of the CKD’s R&D costs recognized as intangible assets.
As of the end of Q3, the hypertension combination drug CKD-828's clinical costs were capitalized at 3.2 billion won.
The development costs of 2.6 billion won and 1.8 billion won, for the dyslipidemia combination drug CKD-391 and the diabetes combination drug CKD-371 were recognized as intangible assets, respectively.
The biosimilar CKD-701, which is a biosimilar of the macular degeneration treatment Lucentis, has capitalized R&D costs of 600 million Korean won.
CKD received approval from the Ministry of Food and Drug Safety in October last year for Lucent-BS, and launched it in Korea in January after receiving health insurance coverage.
Lucent-BS is the second biosimilar released by CKD.
In Nov.
2018, the company received domestic approval for Nesbell, a biosimilar to the anemia treatment NESP.
CKD developed Nesbell after securing a differentiated raw material manufacturing technology in 2008 and establishing biopharmaceutical production infrastructure in 2012.
The amount of R&D costs capitalized by CKD had not been extensive, but its drug pipeline has attracted attention following a recent major technology export deal.
On the 6th of last month, CKD signed a technology export contract with Novartis for the drug candidate CKD-510.
The deal includes a non-refundable upfront payment of US 80 million (106.1 billion won) dollars.
CKD is set to receive milestone payments up to US 1.225 billion (1.6241 trillion won) dollars dependent on development and regulatory approvals.
This was the first major technology export contract the company had signed that exceeded 100 billion won.
CKD-510, which is a new drug candidate developed by CKD, is a highly selective non-hydroxamic acid (NHA) platform technology-based HDAC6 inhibitor.
It has shown efficacy in preclinical studies for various HDAC6 related diseases, including cardiovascular diseases.
It has demonstrated safety and tolerability in Phase 1 clinical trials in Europe and the USA.
CKD has completed a European Phase 1 trial of CKD-510 for Charcot-Marie-Tooth disease (CMT).
CMT is a rare hereditary peripheral neuropathy where genetic mutations lead to motor and sensory nerves damages, complicating normal walking and daily activities.
There is currently no definitive cure for CMT.
CKD’s strategy is to derive optimal drugs for various diseases based on the basic structure of HDAC6 inhibitors.
Currently, CKD is developing new drugs, with a platform technology for CMT, Huntington's disease, Alzheimer's disease, blood cancer, and autoimmune diseases.
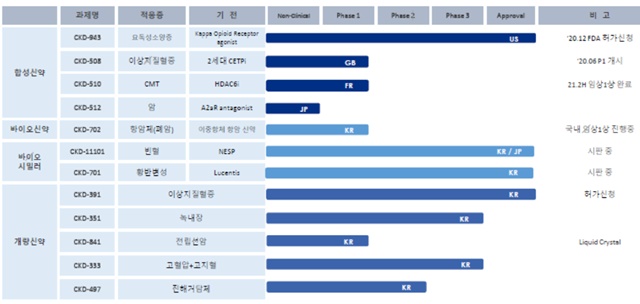
CKD-702 is an anti-cancer bispecific antibody that inhibits both hepatocyte growth factor receptor (c-Met) and epidermal growth factor receptor (EGFR), which are essential for cancer growth.
CKD-702 binds to each receptor to block cancer cell proliferation signals and decrease receptor numbers, making it a first-in-class bio new drug from CKD.
A domestic Phase 1 clinical trial is currently underway.
To verify the efficacy and mechanism of action of CKD-702, CKD conducted research on its use as monotherapy, using non-small cell lung cancer animal models.
The study has shown that CKD-702 exhibits anti-tumor effects by simultaneously inhibiting the hepatocyte growth factor receptor and the epidermal growth factor receptor.
It was also found to be highly effective in animal models that had developed resistance to existing c-Met and EGFR targeted cancer drugs.
Currently, phase 1 trials evaluating its indications for non-small-cell lung cancer are underway, and there are plans to expand the indications to stomach cancer, colon cancer, and liver cancer for global clinical trials.
CKD's new drug, CKD-508, which is being developed as dyslipidemia treatment, has been undergoing Phase 1 clinical trials in the UK since 2020.
The drug is expected to lower LDL cholesterol and increase HDL cholesterol by inhibiting the CETP enzyme.
It is regarded as the most effective CETP inhibitor developed to date, having improved upon the drawbacks of first-generation CETP inhibitors.
CKD aims to complete the Phase 1 clinical trials within the year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









