- LOGIN
- MemberShip
- 2025-12-23 11:42:27
- Marketability of homegrown drugs being tested in the US
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-12-20 05:41:04
Domestic pharmaceutical and bio companies are entering the US one after another.
GC Biopharma succeeded in entering the US market with its blood product on its third try.
Since last year, 3 domestically developed drugs have passed the US market gateway, starting with Hanmi Pharmaceutical and Celltrion.
Until now, homegrown drugs that have entered the U.S.
were deemed to be far from market success.
In this sense, the success potential of homegrown drugs is now being tested in earnest in the US market.
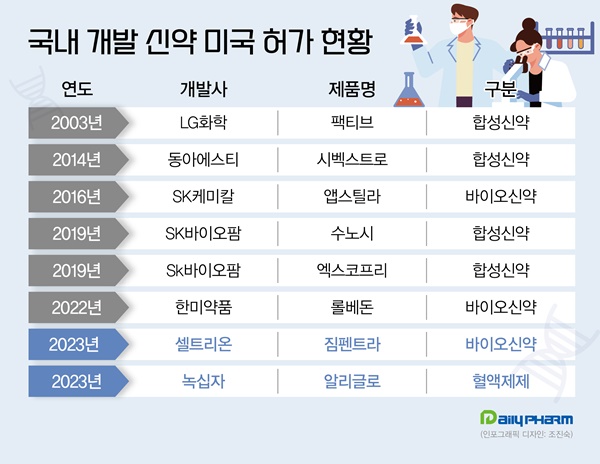
GC Biopharma announced on the 18th that it received marketing authorization for its Alyglo from the US Food and Drug Administration on the 15th.

It is used to treat primary humoral immunodeficiencies such as congenital immune deficiency and Immune thrombocytopenia.
In Korea, the drug is being sold under the brand name, ‘IV-Globulin SN Inj.’ GC Biopharma was able to pass the barrier to US market entry and receive FDA approval on its third try.
GC Biopharma first applied for the approval of its IVIG-SN 5% product at the end of 2015.
In November 2016, the company was asked to supplement the manufacturing process data.
In September 2017, the authorities again requested GC Biopharma to submit supplementary material, delaying approval.
As a result, the company revised its strategy to introduce the more marketable 10% dosage to the U.S.
market first.
In 2020, it completed the North American Phase 3 clinical trial of IVIG-SN 10% Alyglo and submitted a biologics license application to the FDA in February 2021.
However, in February of last year, the company received a notice of delay from the FDA.
Due to the COVID-19 pandemic at the time, the review was conducted non-face-to-face in Q4 2021, but the FDA decided to postpone the drug’s approval due to the need for on-site inspection of the manufacturing facility.
In April, the FDA team conducted an inspection of the manufacturing facility and quality system, including the fractionation and finishing of IVIG-SN at GC Biopharma’s Ochang plant.
After completing the GMP inspection of the Ochang plant, GC Biopharma resubmitted the license application in consultation with the FDA and received final approval this time.
market since last year.
In September last year, the FDA gave the final approval to Spectrum Pharmaceuticals' application for Rolontis (U.S.
brand name Rolvedon)
is administered to prevent or treat neutropenia in cancer patients who receive myelosuppressive chemotherapy in Korea.
In Korea, the drug received approval as the 33rd homegrown novel drug in March 2021.
Rolontis is manufactured at Hanmi’s Pyeongtaek plant.
It is the first Korean biological drug to enter the US market after being produced in a domestic plant that passed the FDA’s on-site inspections.
Rolvedon became the 6th new drug developed by a domestic company to clear the FDA approval process.
In October, Celltrion received approval from the US FDA to market Zymfentra, a subcutaneous (SC) formulation of its antibody biosimilar Remsima as a new drug.
Remsima is a biosimilar product of Remicade.
Zymfentra is licensed and marketed in Europe under the name Remsima SC.
Zymfentra is Celltrion's first product licensed as a new drug in the United States.
To obtain approval as a new drug, Celltrion conducted two new global Phase III clinical trials.
Based on the safe safety and efficacy results demonstrated through the clinical trial Celltrion submitted an application for approval in December of last year in accordance with the FDA's new drug approval process and obtained approval in 10 months.
LG Chem’s antibiotic Factive was the first among homegrown new drugs to pass the US gates in 2003.
Then, Sivextro that Dong-A ST licensed-out was approved by the FDA in 2014.
Then, in 2016, SK Chemical’s hemophilia drug Afstyla received FDA approval.
Afstyla is a new genetic recombinant drug that was developed by SK Chemical with its proprietary technology.
SK Chemical licensed the drug candidate to Australia’s CSL Behring in the preclinical stage in 2009, and CSL Behring conducted the clinical trials and received approval for the drug in the US and Europe.
In 2019, two of SK Biopharmaceuticals’ drugs - the narcolepsy drug Sunosi and the new epilepsy drug Xcopri - received FDA approval.
In 2019, Sunosi, SK Biopharmaceuticals’ sleep disorder drug that the company licensed out, received final approval from the FDA.
SK Biopharmaceuticals completed Phase I trials for the candidate drug after discovery and licensed-out the candidate Jazz Pharmaceuticals in 2011.
Jazz acquired the global commercialization rights for Sunosi, including those for the US and Europe, completed Phase III trials, and reached the commercialization stage.
In November 2019, the company also received FDA approval for its epilepsy treatment Xcopri.
Xcopri is the first new drug to be solely developed and applied and granted FDA approval by a domestic company.
Xcopri is a positive allosteric modulator of the y-aminobutyric acid (GABAA) ion channel, which blocks the voltage-gated sodium currents, causing the reduction in repetitive neuronal firing, and reducing seizures.
As such, the industry believes this is the time to test the potential for commercial success of homegrown drugs in the United States.
So far, domestic drugs that have received US approval have been far from conquering the global stage.
Factive’s overseas expansion was hindered when its partner GlaxoSmithKline withdrew from the deal over clinical data.
More than KRW 300 billion was invested in its development, but its sales in the U.S.
have been negligible.
Sivextro, which was approved by the FDA in 2014, and Afstyla, which debuted in the U.S.
market in 2016, have not performed as well commercially as expected.
The company voluntarily withdrew the license for Sivextro, citing low drug prices in Korea.
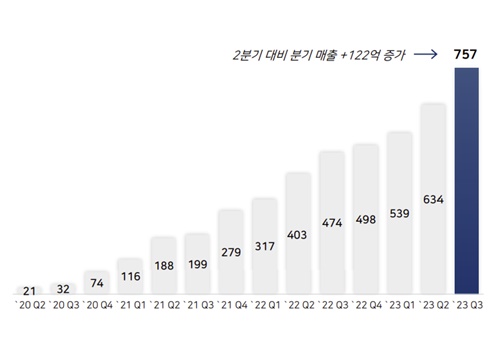
Since its launch in the US, Xcopri has continued to renew its sales record every quarter.
After posting initial sales of KRW 2.1 billion in the US in 2020, the drug generated sales of KRW 75.7 billion in Q3 this year.
Xcopri’s cumulative sales in the US last year were KRW 169.2 billion, and in Q3 of this year alone, Xcopri surpassed last year's sales with KRW 193 billion.
Xcopri’s cumulative sales in the U.S.
over the past 3 years totaled at KRW 452.1 billion.
Rolvedon, a drug licensed out by Hanmi Pharmaceutical, has also continued to grow in the US market, with sales of $15.6 million (KRW 20 billion) and $21 million (KRW 28 billion) in Q1 and Q2 respectively.
Rolvedon was developed by Assertio Holdings, a pharmaceutical company specializing in central nervous system, pain, and inflammation, which acquired Spectrum Pharmaceuticals in April.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









