- LOGIN
- MemberShip
- 2025-12-23 07:53:32
- Generic companies win 3 out 4 patent disputes
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-12-27 11:39:43
This year, out of 29 trial decision and verdict cases that arose as patent disputes, companies that filed a trial or lawsuit have won in 21 cases.
This translates to a win rate of approximately 76% for companies challenging patents.
Especially, excluding cases where the lawsuit or suit qualification was deemed ineligible, the dispute hadn’t commenced, or was dismissed, generic companies have won all the other disputes, except for the Dukarb dispute.
This year, patent challenging companies have won 22 out of 29 trial decision and verdict cases According to the pharmaceutical industry on the 26th, 29 cases of trial decisions and verdicts were made on patent disputes, excluding those cases with voluntary withdrawal of trial decisions or lawsuits.
The 21 patent dispute cases included trial decisions from the first trial at the Intellectual Property Trial and Appeal Board (IPTAB), 5 verdicts from the second trial at the Patent Court of Korea, 3 verdicts from the third trial at the Supreme Court of Korea.
Out of the 29 cases, 22 were won by companies that filed for the trial decisions or lawsuit.
In terms of pharmaceutical patents, of the 4 cases that reached final decisions or lawsuits, 3 of which (76%) ended in victory.
Excluding instances where cases were dismissed because the requirements for a patent trial claim were not met, or cases where the patent-holder deleted the related claim phrase after the trial has begun, generic companies have scored a major victory in all disputes, except for the Dukarb dispute.
When generic companies challenge a patent, they typically approach it with a fully developed abdication or invalidating plan; therefore, this approach is known to contribute to a high success rate in the first trial.
In other words, it’s relatively rare for companies challenging patents to lose in the first trial.
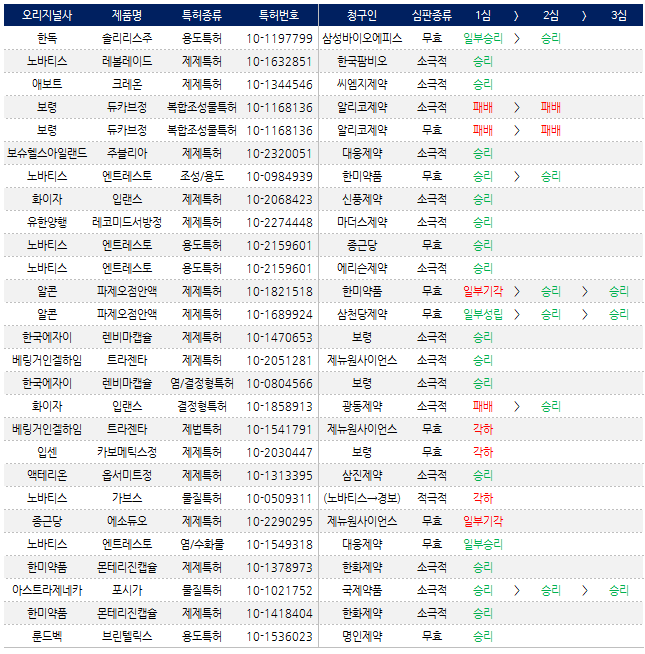
In Jaunary 2023, the IPTAB ruled in favor of the original-drug developer, dismissing the trial decision regarding Arlico pharm and others who filed a patent invalidation trial against Boryung Pharmaceutical’s combination drug patent related to Dukarb.
Boryung also secured a victory in the second trial.
In November, the patent court of Korea ruled against the plaintiffs in two cases of Dukarb patent disputes, favoring Boryung.
These cases are awaiting a final ruling by the Supreme Court, following an appeal by generic companies who lost in the second trials.
Boryung, having won both first and second trials, has been able to maintain the patent for Dukarb key dosages (30/5mg).
As a result, generic companies are unable to release generics of these key dosages onto the market.
Disputes involving Forxiga and Pazeo Eye Drops have finally concluded, with generic companies securing victory The two disputes involving SGLT-2 class diabetes treatments, Forxiga and Pazeo Eye Drops, have finally concluded with the Supreme Court ultimately ruling in favor of the generic companies that challenged patents.
In February 2023, the Supreme Court issued a final ruling on the patent dispute regarding Forxiga, concluding a patent dispute that spanned eight years.
The dispute comes after Kukje Pharma and other Korean companies filed a patent invalidation trial of the 2nd substance patent.
The generic companies won both the first and second trials.
AstraZeneca then appealed to the Supreme Court.
However, in February, the Supreme Court ultimately ruled in favor of the generic companies.
Following their victory in the lawsuit, generic companies could advance their plans by 9 months.
After the 1st substance patent voided on April 7th 2023, generics released their drugs onto the market.

The generic companies secured an ultimate victory after a six-year-long patent dispute.
The dispute started on June 2017, when SamChunDang Pharm filed a patent invalidation trial against an active ingredient patent.
Then in the following year, Hanmi Pharm filed a patent invalidation trial in another active ingredient patent.
Having lost in the first trial, generic companies won all cases of the lawsuits.
Novartis then appealed to the court, but the generic companies ultimately won the cases.
Generic companies win patent disputes of Jublia, Recomid, and Monterizine The patent disputes concerning Jublia Topical Solution, Recomid SR Tab., Monterizine Cap., Ibrance Cap., Lenvima Cap., and Opsumit Tab.
reached decisions in the first trials.
In the patent dispute involving Jublia, seven companies, including Daewoong Therapeutics, gained victories in the first trial in November.
In a similar lawsuit, ten pharmaceutical companies, including, Jeil Pharm, are still awaiting a decision.
Yet, pharmaceutical companies anticipate that generic companies will likely win the cases, in line with previous trial decisions.

in October.
Last year, when generic companies filed for patent lawsuits, the reimbursement status of Rebamipide ingredient, which includes Recomid SR Tab., was uncertain because those were included as candidates in the 2023 reimbursement re-evaluation review.
This year, however, Rebamipide successfully passed the reimbursement re-evaluation review.
Additionally, the companies that challenged patents have also won in related trials, thus increasing the likelihood of early releases of generics.
Companies like Han Wha Pharma achieved victory in trial decisions on four cases related to passive trials to confirm the scope of a right involving Hanmi Pharm’s Monterizine Cap.
active ingredient patent.
Although Hanmi Pharm, the original-drug developer, appealed to the patent court of Korea for two out of these four cases, the disputes ended with its withdrawal.
Companies like Shin Poong Pharmaceutical, Boryung Pharmaceutical, and Samjin Pharmaceutical have achieved victories in passive trial to confirm the scope of a right.
Shin Poong Pharmaceutical won against Pfizer's Ibrance active ingredient patent in October, Boryung Pharmaceutical Eisai's Lenvima active ingredient patent and salt and crystalline form patent in August, and Samjin Pharmaceutical won Actelion's Opsumit active ingredient patent in April.
Novartis faced defeat in passive trial to confirm the scope of a right involving Gabes ingredient patent filed against Kyongbo Pharmaceutical.
The Patent Court of Korea ruled against Novartis in April of this year.
Despite Novartis previously initiating patent litigation against generic companies, these efforts did not achieve the desired outcomes.
In January of the previous year, Novartis had filed a passive trial to confirm the scope of a right with the Patent Court of Korea to ascertain whether Kyongbo Pharmaceutical had infringed on the Gabes ingredient patent.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










