- LOGIN
- MemberShip
- 2025-12-23 07:55:35
- KRW 250 bil market shakes from reimb reevaluations
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-12-28 06:02:26
The pharmaceutical industry has expressed great disappointment regarding the results of the government’s drug reimbursement reevaluations.
The reimbursement scope of three ingredients - loxoprofen, limaprost alpha dex, and epinastine hydrochloride - will be reduced.
This is a loss worth KRW 250 billion in the prescription market.
The losses felt by pharmaceutical companies are expected to be even greater due to the reimbursement reductions made for products that have seen a surge in their respective prescription markets due to the COVID-19 pandemic and endemic.
This year's reimbursement reevaluations resulted in the indication reduction of 3 substances including loxoprofen...
KRW 250 billion market impact According to industry sources on the 28th, the Ministry of Health and Welfare recently held a Health Insurance policy review committee and announced the results of this year's salary reassessment.
Reimbursement for the 2 substances, rebamipide, and levosulpiride, which demonstrated their clinical utility, was decided to be maintained as is.
Three substances including loxoprofen sodium, limaprost alpha dex, and epinastine hydrochloride, will have their coverage reduced.
Evaluation of 2 substances, oxiracetam, and acetyl-L-carnitine hydrochloride, whose reimbursement has already been discontinued and efficacy/effect indications removed due to lack of evidence of efficacy during MFDS clinical reevaluations, were excluded from reimbursement reevaluations.

The list includes a number of products that have seen a surge in prescription volume in the wake of the recent COVID-19 pandemic and epidemic.
According to the market research institution UBIST, the total outpatient prescription market size of the three benefit reductions - loxoprofen, rimaprost alpadex, and epinastine - was KRW 251.4 billion last year.
This is a large market worth 250 billion won a year, a market where a decline in prescriptions is expected with the reimbursement reduction.
Loxoprofen’s reimb as a pain and reliever indication deleted from specifications…lights can serve as a loss of a KRW 100 million. Loxoprofen has been reimbursed in three areas: ▲as analgesia for chronic rheumatoid arthritis, osteoarthritis (degenerative arthritis), low back pain, periarthritis of the shoulder, and cervical spondylosis; ▲ analgesia for postoperative, post-traumatic, and post-extraction pain, ▲ and as antipyretic analgesia for acute upper respiratory tract infections.
Of these, the ‘antipyretic analgesia for acute upper respiratory tract infection' indication will be removed as after the government found its reimbursement justification inadequate.
For pharmaceutical companies, the removal of one indication for loxoprofen will result in lost prescriptions.
Last year, the outpatient prescription market for loxoprofen was estimated at 103.5 billion won.
Given the rapid growth of loxoprofen’s reimbursement-deleted indication during the COVID-19 pandemic, the losses felt by the pharmaceutical companies are expected to be even greater.
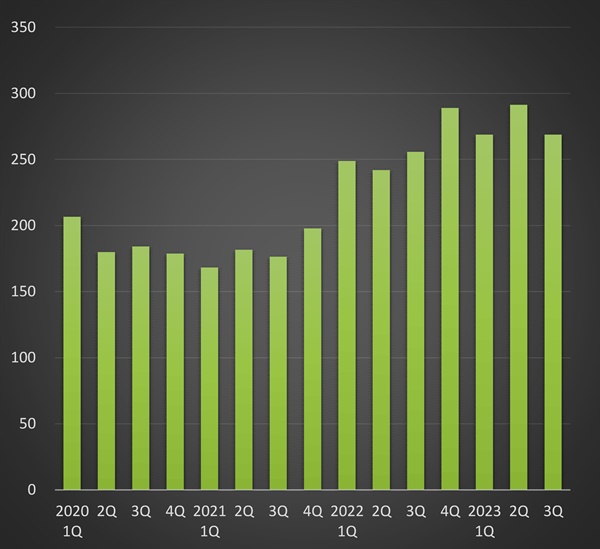
However, the prescription market for loxoprofen surged 43.0% year-on-year to KRW 103.5 billion last year.
Since late 2021, the demand for loxoprofen has increased dramatically when the number of COVID-19 cases increased by hundreds of thousands per day.
Loxoprofen continued to show growth in sales this year.
the growth trend has continued.
In the third quarter, prescriptions for loxoprofen totaled KRW 26.9 billion, up 52.4% from two years ago.
Cumulative prescriptions in the third quarter of this year showed that the amount totaled KRW 82.9 billion, up 57.5% in 2 years from the KRW 52.6 billion it had raised in December 2021.
of 2021.
The recent increase in prescriptions for loxoprofen is likely due to the increase in cold and flu patients since the end of the COVID-19 pandemic.
KRW 150 bil market may contract due to reduced reimbursement of limaprost alpha dex and epinastine limaprost alpha dex is used to ▲improve ischemic symptoms such as ulcers, arthralgia, and coldness caused by occlusive thromboangiitis, and to ▲improve self-symptoms and walking ability caused by acquired lumbar spinal stenosis.
Dong-A ST’s Dong-A Opalmon is the original drug.
limaprost alpha dex’s reimbursement for one of the two of its indications, ‘Improvement of ischemic symptoms such as ulcers, arterial pain, and coldness caused by occlusive thromboangiitis’ will be excluded from reimbursement.
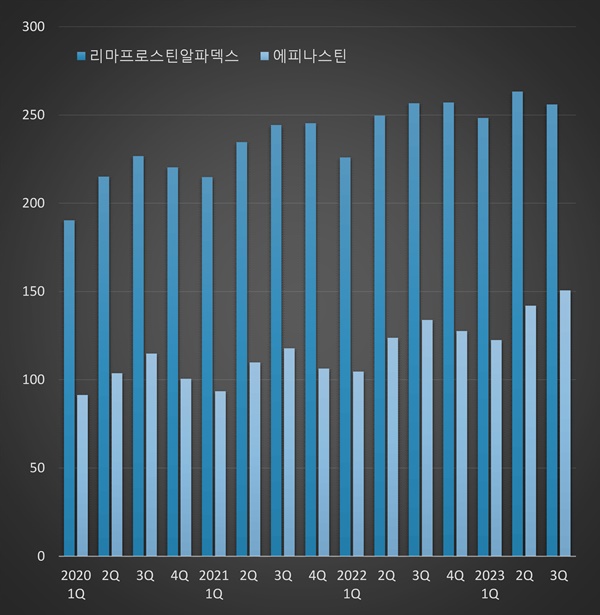
Prescription sales of limaprost alpha dex had recorded over KRW 25.6 billion in Q3.
This is a 12.9% three-year increase from the KRW 22.7 billion in Q3 2020.
While growth in the prescription market for limaprost may not have been significant, the removal of one of the two indications is likely to contract the prescription market.
Last year, prescriptions for limaprost alpha dex amounted to KRW 98.9 billion.
Epinastine is indicated for ▲ bronchial asthma ▲ allergic rhinitis ▲ urticaria, eczema-dermatitis, itchy skin, itchy rash, moderate psoriasis with itching ▲ prevention and relief of itching in allergic conjunctivitis.
This indication, "Prevention and relief of itching in allergic conjunctivitis," is applicable only to epinastine ophthalmic solutions.
Epinastine’s outpatient prescription sales in Q3 were KRW 15.1 billion.
This is a 12.5% increase from the same period last year and a 27.9% increase from KRW 11.8 billion in Q3 2021.
The increase in prescriptions for epinastine can be attributed to its increased use for bronchial asthma since the COVID-19 outbreak.
With the removal of epinastine’s bronchial asthma indication reimbursement, prescription sales are expected to decline.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










