- LOGIN
- MemberShip
- 2025-12-23 06:04:30
- Acetaminophen syrup receives price hikes to meet demand
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-12-28 06:02:42
Starting next month, the maximum reimbursement prices for two acetaminophen suspension formulations will be increased by over 50%.
The price hike follows the government’s decision to implement measures aimed at encouraging pharmaceutical companies to expand volume of acetaminophen production.
Currently, there is a continuous demand for the drugs due to a high number of patients with influenza and the common cold.
Following the drug price increase for pill-type acetaminophen monotherapy last year, suspension formulations have now joined the list of drug receiving pricing increases within a year.
The drug pricing increase in acetaminophen suspension formulations is expected to lead to an annual increase by approximately 1 billion won in prescription sales.
According to the Ministry of Health and Welfare (MOHW) on 27th, starting next month, the prices of the two acetaminophen suspension formulations will be increased by up to 55.6%.
The price of Janssen Korea’s Children's Tylenol Suspension will be increased by 55.6%, adjusted from 18 won to 28 won, and Sama Pharm’s Setopen Suspension will be increased by 52.9%, adjusted from 17 won to 26 won.
During the COVID-19 pandemic and endemic, the demand for acetaminophen, an antipyretic drug, has significantly increased.
However, the low drug price has made it difficult to expand the production.
In response, the government has decided to bring an increase in drug pricing to encourage drug companies to expand their volume of production.
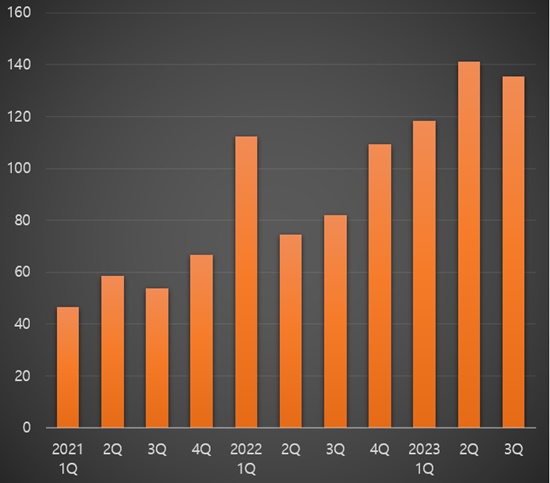
According to the data from UBIST, a drug market research agency, cumulative outpatient prescription sales in Q3 this year was1.9 billion won, a significant increase of 94.9% compared to the same period last year.
When compared to the cumulative prescription sales of 4 billion won in Q3 2021, this is a five-fold expansion in just two years.
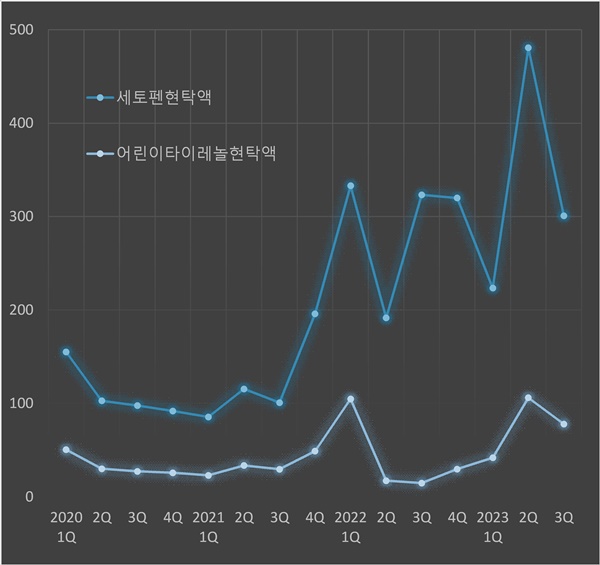
The cumulative prescription sales of Setopen Suspension in Q3 were 1.6 billion won, a five times increase in just two years.
While the cumulative prescription sales of Children's Tylenol Suspension in Q3 of 2021 were below 100 million won, they have now reached 300 million won, showing more than three times increase.
The quarterly prescription sales of acetaminophen suspension formulations have increased by three times, in particular, prescription sales of Setopen Suspension in Q3 of 2021 was merely 100 million won, but, this year’s YoY sales was 300 million won.
In the same line, the prescription sales of last year’s Q2 was nearly 500 million won, which is three-fold expansion compared to two years ago.
The prescription sales of Children's Tylenol Suspension in Q1 recorded below 100 million won, but, in Q2, it surpassed 100 million won.
The prescription sales of Children's Tylenol Suspension in Q3 were only 70,000,000 won, but compared to two years ago, it has increased by more than two-fold.
The market size of suspension formulations is larger than the entire market for acetaminophen monotherapy.
The cumulative prescription sales of acetaminophen monotherapy in Q3 of 2021 reached 39.5 billion won, a 148.5% hike from the same period of last year.
The margin of increase in prescription sales for suspension formulations was even greater, approximately two times higher.
The MOHW raised the upper limit for 18 items by up to 76.5%, starting in December 2022.
The maximum reimbursement price of acetaminophen 650mg was only 43-51 won, but it has been adjusted to 90 won.
The government decided to collectively set the price hike considering that many pharmaceutical companies were reluctant to expand the volume of production due to the poor cost structure.
The pharmaceutical companies have agreed to expand production along with the increased drug pricing of acetaminophen.
The price of Janssen Korea’s Tylenol 8 hours was adjusted from 51 won to 90 won, recording the highest increase rate of 76.5%.
Both Bukwang Pharm’s Tacenol 8hours and Chong Kun Dang Pharmaceutical’s PENZAL were adjusted from 51 won to 88 won, a 72.5% hike.
Hanmi Pharm’s Suspen 8 hours adjusted from 50 won to 85 won, a 70% hike.
Kolon Pharmaceutical’s Tramol 8 hours is set to increase from 51 won to 85 won, a 66.7% hike.
Genu Pharma’s Anisphen 8 hours and Hana Pharm’s Tylicol 8 hours will both be adjusted to 83 won, a 62.7% hike.
Sama Pharm’s Setopen and Young Poong Pharmaceutical’s Tifen 8 hours are set to increase from 51 won to 80 won, a 56.9% hike.
The adjusted prices of eight items, including Boryung Biopharma’s Cetaphen 8hr, will be raised to approximately 70 won.
Initially, the price of acetaminophen 650g was schedule to be collectively adjusted to 70 won starting in December, but the price adjustment will be delayed until March of next year.
The recent prescription sales for a year (Q4 of 2022 to Q3 of 2023) of Setopen Suspension and Children's Tylenol Suspension were reported to be 1.3 billion won and 300 million won, respectively.
Applying the price increase rates for Setophen Suspension and Children's Tylenol Syrup, the annual prescription sales hike is expected to be 2 billion won and 400 million won, respectively.
As a result of the price increase for these two acetaminophen syrup formulations, an expansion of the prescription market by 800 million won annually is anticipated.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










